Home /
Expert Answers /
Chemistry /
a-steel-structure-will-be-cathodically-protected-by-sacrificial-anodes-a-illustrate-the-princip-pa864
(Solved): A steel structure will be cathodically protected by sacrificial anodes. (a) Illustrate the princip ...
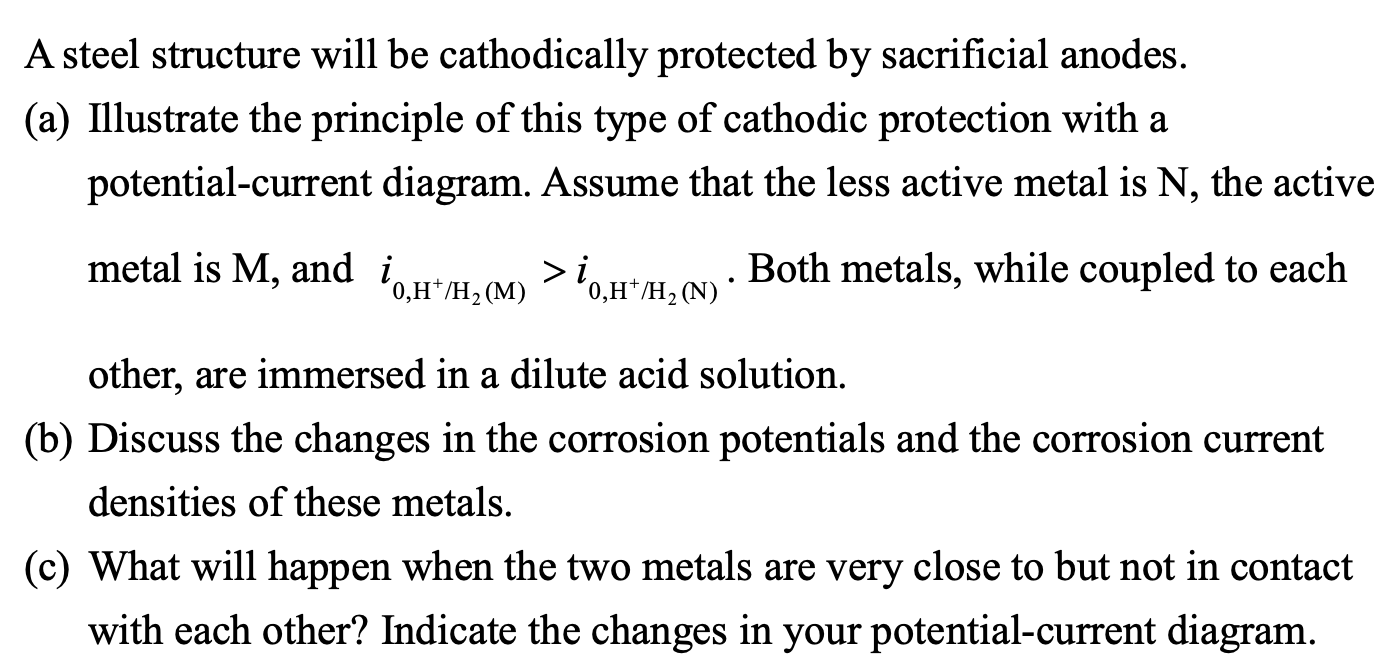
A steel structure will be cathodically protected by sacrificial anodes. (a) Illustrate the principle of this type of cathodic protection with a potential-current diagram. Assume that the less active metal is \( \mathrm{N} \), the active metal is \( \mathrm{M} \), and \( i_{0, \mathrm{H}^{+} / \mathrm{H}_{2}(\mathrm{M})}>i_{0, \mathrm{H}^{+} / \mathrm{H}_{2}(\mathrm{~N})} \). Both metals, while coupled to each other, are immersed in a dilute acid solution. (b) Discuss the changes in the corrosion potentials and the corrosion current densities of these metals. (c) What will happen when the two metals are very close to but not in contact with each other? Indicate the changes in your potential-current diagram.
Expert Answer
HERE IS YOUR ANSWER A-> The principle of cathodic protection using sacrificial anodes can be illustrated with a potential-current diagram as follows: 1-> Two metals (N and M) connected to one another and submerged in a weak acid solution will both co