Home /
Expert Answers /
Chemistry /
a-solution-of-permanganate-is-standardized-by-titration-with-oxalic-acid-h2c2o4-it-req-pa938
(Solved): A solution of permanganate is standardized by titration with oxalic acid (H2C2O4). It req ...
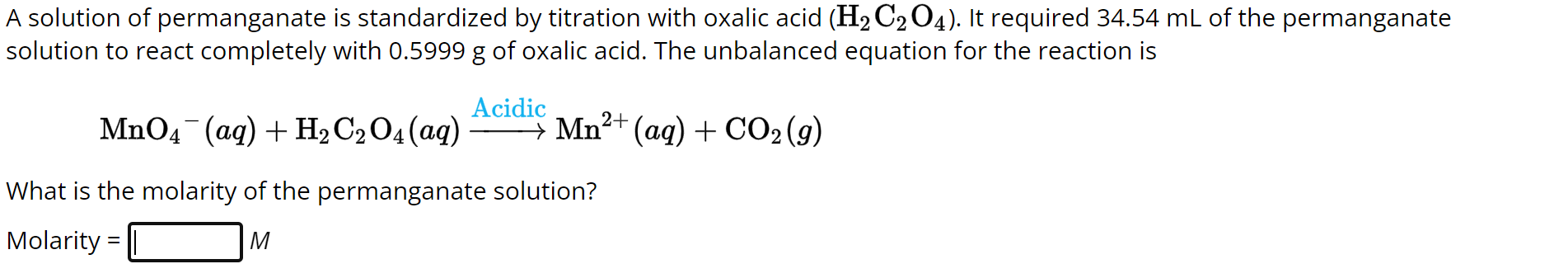

A solution of permanganate is standardized by titration with oxalic acid . It required of the permanganate solution to react completely with of oxalic acid. The unbalanced equation for the reaction is What is the molarity of the permanganate solution?
The vanadium in a sample of ore is converted to . The ion is subsequently titrated with in acidic solution to form and manganese(II) ion. To titrate the solution, of was required. If the mass percent of vanadium in the ore was , what was the mass of the ore sample? Mass
Expert Answer
1.Balanced chemical equation :- 5 moles of req...