Home /
Expert Answers /
Chemistry /
a-scientist-measures-the-standard-enthalpy-change-for-the-following-reaction-to-be-2923-8-math-pa972
(Solved): A scientist measures the standard enthalpy change for the following reaction to be \( 2923.8 \math ...
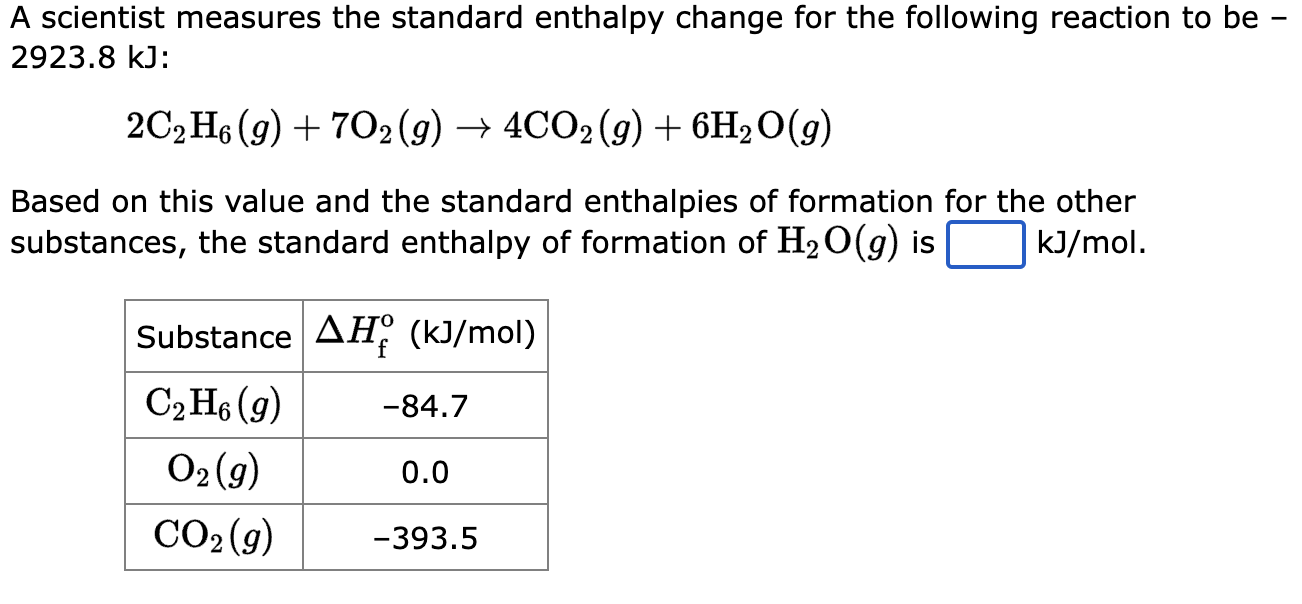
A scientist measures the standard enthalpy change for the following reaction to be \( 2923.8 \mathrm{~kJ} \) : \[ 2 \mathrm{C}_{2} \mathrm{H}_{6}(g)+7 \mathrm{O}_{2}(g) \rightarrow 4 \mathrm{CO}_{2}(g)+6 \mathrm{H}_{2} \mathrm{O}(g) \] Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of \( \mathrm{H}_{2} \mathrm{O}(g) \) is \( \mathrm{kJ} / \mathrm{mol} \).