Home /
Expert Answers /
Chemistry /
a-sample-of-solid-biphenyl-weighing-0-5260g-was-ignited-in-a-bomb-calorimeter-initially-at-25-pa244
(Solved): A sample of solid biphenyl weighing 0.5260g was ignited in a bomb calorimeter initially at 25 ...
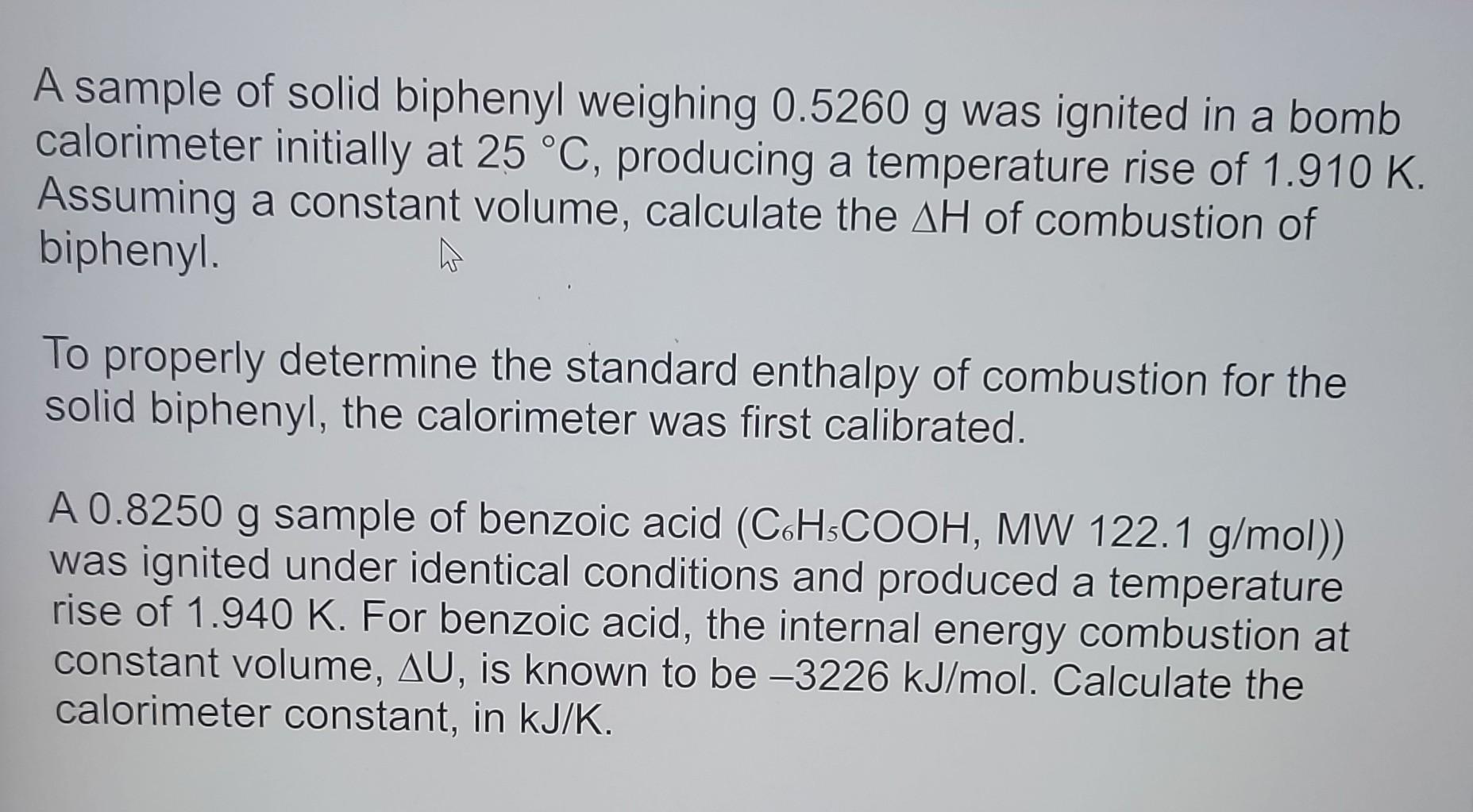
A sample of solid biphenyl weighing was ignited in a bomb calorimeter initially at , producing a temperature rise of . Assuming a constant volume, calculate the of combustion of biphenyl. To properly determine the standard enthalpy of combustion for the solid biphenyl, the calorimeter was first calibrated. A sample of benzoic acid was ignited under identical conditions and produced a temperature rise of . For benzoic acid, the internal energy combustion at constant volume, , is known to be . Calculate the calorimeter constant, in .
Expert Answer
Please Upvote dear! I badly need it. Thanks in advance.Solution 1:The following formula may be used ...