Home /
Expert Answers /
Chemistry /
a-sample-of-solid-benzoic-acid-c-6-h-5-c-o-o-h-that-weighs-0-800-g-is-burned-in-an-excess-of-pa940
(Solved): A sample of solid benzoic acid (C_(6)H_(5)(C)/(O)O H) that weighs 0.800 g is burned in an excess of ...
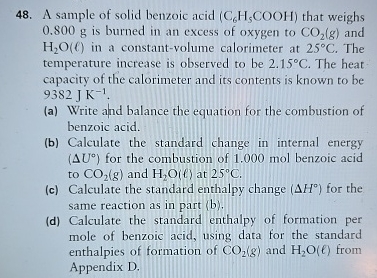
A sample of solid benzoic acid
(C_(6)H_(5)(C)/(O)O H)that weighs 0.800 g is burned in an excess of oxygen to
CO_(2)(g)and
H_(2)O(l)in a constant-volume calorimeter at
25\deg C. The temperature increase is observed to be
2.15\deg C. The heat capacity of the calorimeter and its contents is known to be
9382JK^(-1). (a) Write and balance the equation for the combustion of benzoic acid. (b) Calculate the standard change in internal energy
(\Delta U\deg )for the combustion of 1.000 mol benzoic acid to
CO_(2)(g)and
H_(2)O(l)at
25\deg C. (c) Calculate the standard enthalpy change
(\Delta H\deg )for the same reaction as in part (b). (d) Calculate the standard enthalpy of formation per mole of benzoic acid, using data for the standard enthalpies of formation of
CO_(2)(g)and
H_(2)O(l)from Appendix D.