Home /
Expert Answers /
Chemistry /
a-sample-of-potassium-dichromate-k2cr2o7-contains-4-2-x-1024-atoms-of-oxygen-how-many-atoms-of-ch-pa339
(Solved): A sample of potassium dichromate, K2Cr2O7, contains 4.2 x 1024 atoms of oxygen. How many atoms of ch ...
A sample of potassium dichromate, K2Cr2O7, contains 4.2 x 1024 atoms of oxygen. How many atoms of chromium are in the sample? [? ?] x 10 x 10²¹ atoms Cr Enter the coefficient in the green blank and the exponent in the yellow blank. Report your answer to the appropriate number of significant figures! Exponent (yellow) Coefficient (green) Enter
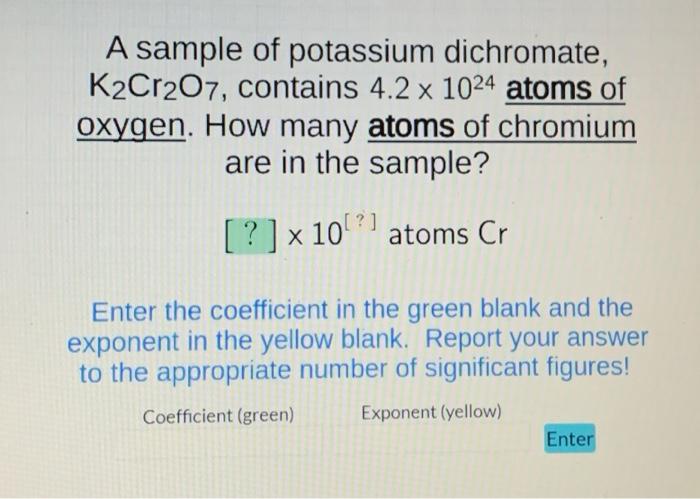
A sample of potassium dichromate, , contains atoms of oxygen. How many atoms of chromium are in the sample? Enter the coefficient in the green blank and the exponent in the yellow blank. Report your answer to the appropriate number of significant figures! Coefficient (green) Exponent (yellow)