Home /
Expert Answers /
Chemistry /
a-sample-of-naphthalene-is-bumed-in-a-bomb-calorimeter-containing-257-grams-of-water-at-an-initial-pa929
(Solved): A sample of naphthalene is bumed in a bomb calorimeter containing 257 grams of water at an initial ...
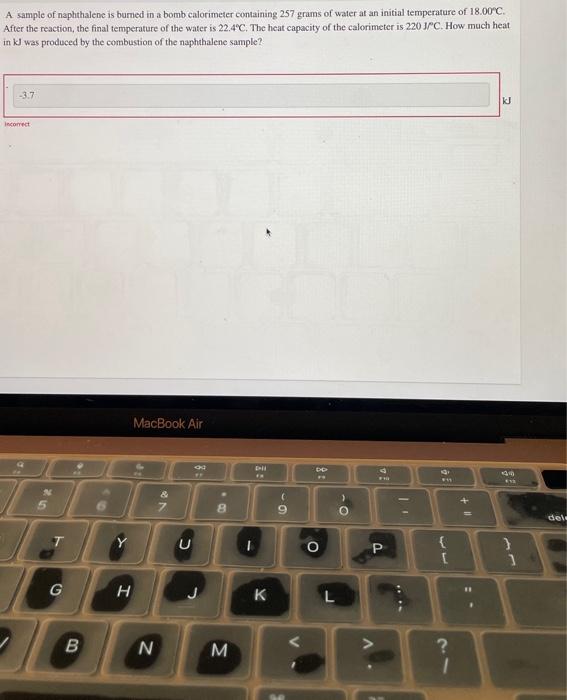
A sample of naphthalene is bumed in a bomb calorimeter containing 257 grams of water at an initial temperature of . After the reaction, the final temperature of the water is . The heat capacity of the calorimeter is . How much heat in was produced by the combustion of the naphthalene sample?