Home /
Expert Answers /
Chemistry /
a-reaction-mixture-initially-containing-vitamin-c-at-0-0112-m-h202-at-0-14-m-and-iodide-ion-at-0-0-pa555
(Solved): A reaction mixture initially containing vitamin C at 0.0112 M, H202 at 0.14 M, and iodide ion at 0.0 ...
A reaction mixture initially containing vitamin C at 0.0112 M, H202 at 0.14 M, and iodide ion at 0.03 M. The mixture turns from colorless to dark blue-black after 24.7 seconds. Calculate the rate of this reaction if the final iodide concentration is 0.013 M.
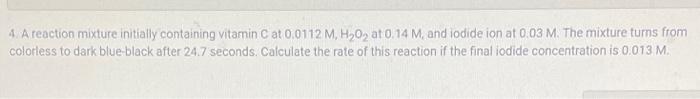
4. A reaction mixture initially containing vitamin \( \mathrm{C} \) at \( 0.0112 \mathrm{M}, \mathrm{H}_{2} \mathrm{O}_{2} \) at \( 0.14 \mathrm{M} \), and iodide ion at \( 0.03 \mathrm{M} \). The mixture tums from colorless to dark blue-black after \( 24.7 \) seconds. Calculate the rate of this reaction if the final iodide concentration is \( 0.013 \mathrm{M} \).
Expert Answer
Given, Concentration of vitamin C = 0.0112 M Concentration of H2O2 = 0.14 M. Concentration of iodide ion = 0.03 M The mixture turns fro