Home /
Expert Answers /
Chemistry /
a-reaction-is-performed-to-study-the-reaction-of-mercury-ii-chloride-with-oxalate-ion-2-mathr-pa137
(Solved): A reaction is performed to study the reaction of mercury(II) chloride with oxalate Ion: \[ 2 \mathr ...
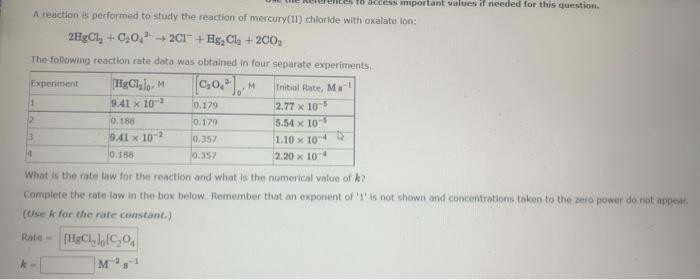
A reaction is performed to study the reaction of mercury(II) chloride with oxalate Ion: \[ 2 \mathrm{HgCl}_{2}+\mathrm{C}_{2} \mathrm{O}_{4}^{2-}+2 \mathrm{Cl}^{-}+\mathrm{Hg}_{2} \mathrm{Cl}_{2}+2 \mathrm{CO}_{2} \] The following reaction rate data was obtained in four separate experiments. What is the rate law for the reaction and what is the numerical value of \( k \) ? Complete the rate law in the box below. Remember that an exponent of '1' is not shown and concentrations taken to the zero pewer do not apperf, (Qae k for the rafe constant.) Rafe