Home /
Expert Answers /
Chemistry /
a-precipitation-reaction-involves-the-formation-of-a-precipitate-when-aqueous-solutions-are-mixed-pa769
(Solved): A precipitation reaction involves the formation of a precipitate when aqueous solutions are mixed. ...
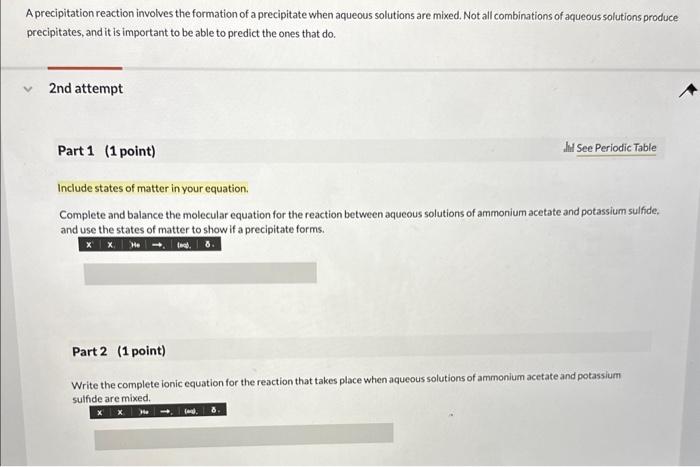

A precipitation reaction involves the formation of a precipitate when aqueous solutions are mixed. Not all combinations of aqueous solutions produce precipitates, and it is important to be able to predict the ones that do. 2nd attempt Part 1 (1 point) Whi See Periodic Table Include states of matter in your equation. Complete and balance the molecular equation for the reaction between aqueous solutions of ammonium acetate and potassium sulfide, and use the states of matter to show if a precipitate forms. Part 2 (1 point) Write the complete ionic equation for the reaction that takes place when aqueous solutions of ammonium acetate and potassium sulfide are mixed.
Write the net ionic equation for the precipitation reaction, if any, that may occur when aquecus solutions of ammonium acetate and potassium sulfide are mixed, Include states of matter. If there is no net ionic equation, simply write "none"
Expert Answer
An equation is called balanced when th