Home /
Expert Answers /
Chemistry /
a-practice-balance-the-following-reactions-and-label-the-physical-state-for-each-compound-in-the-pa386
(Solved): A. Practice: Balance the following reactions and label the physical state for each compound in the ...
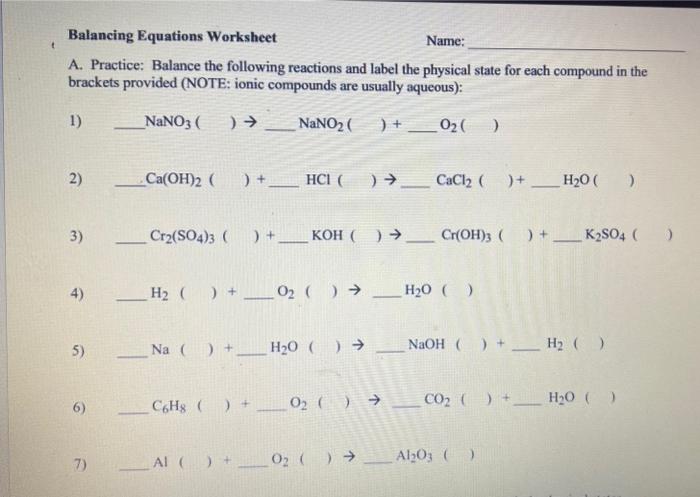

A. Practice: Balance the following reactions and label the physical state for each compound in the brackets provided (NOTE: ionic compounds are usually aqueous): 1) \( \mathrm{NaNO}_{3}(\longrightarrow) \rightarrow \mathrm{NaNO}_{2}(\longrightarrow)+\mathrm{O}_{2}(\quad) \) 2) \( \quad \mathrm{Ca}(\mathrm{OH})_{2}(\quad)+\ldots \mathrm{CaCl}_{2}(\quad)+\mathrm{HCl}_{2} \mathrm{O}(\quad) \) 3) \( \mathrm{Cr}_{2}\left(\mathrm{SO}_{4}\right)_{3}(\quad)+\ldots \mathrm{KOH}(\quad) \rightarrow \ldots \mathrm{Cr}(\mathrm{OH})_{3}(\quad)+\mathrm{K}_{2} \mathrm{SO}_{4}( \) ) 4) 5) \( \mathrm{Na}(\mathrm{O})+\mathrm{H}_{2} \mathrm{O}(\mathrm{O}) \rightarrow \mathrm{NaOH}_{(}(\mathrm{O})+\mathrm{H}_{2}(\quad) \) 6) \( \mathrm{C}_{6} \mathrm{H}_{8}()+\mathrm{O}_{2}() \rightarrow \mathrm{CO}_{2}()+\mathrm{H}_{2} \mathrm{O}() \)
B. Translate the following sentences into balanced chemical equations. Include physical states. 8) Sulfur trioxide gas reacts with water to form sulfuric acid solution. 9) Sodium metal reacts with chlorine gas to form table salt. 10) Solid ammonium nitrate will break down into dinitrogen monoxide gas and water.