Home /
Expert Answers /
Chemistry /
a-neutral-atom-has-the-following-electron-configuration-kr-5s-2-4d-10-5p-5-what-is-the-chemica-pa211
(Solved): A neutral atom has the following electron configuration: [Kr]5s^(2)4d^(10)5p^(5) What is the chemica ...
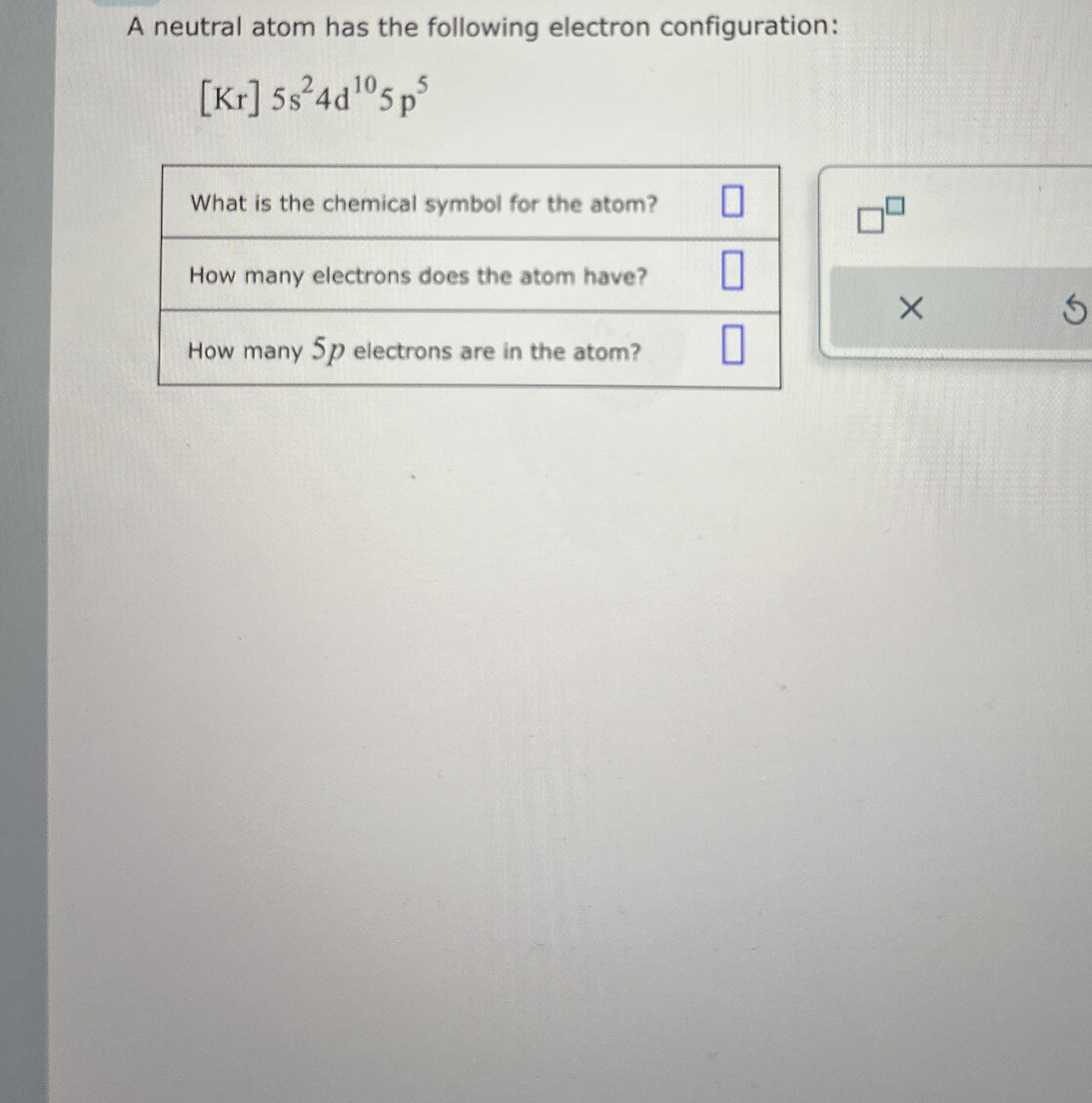
A neutral atom has the following electron configuration:
[Kr]5s^(2)4d^(10)5p^(5)What is the chemical symbol for the atom? How many electrons does the atom have? How many 5 pelectrons are in the atom?