Home /
Expert Answers /
Chemistry /
a-naturally-occurring-element-consists-of-two-isotopes-one-isotope-is-31-abundant-and-has-pa235
(Solved): A naturally occurring element consists of two isotopes. One isotope is \( 31 \% \) abundant and has ...
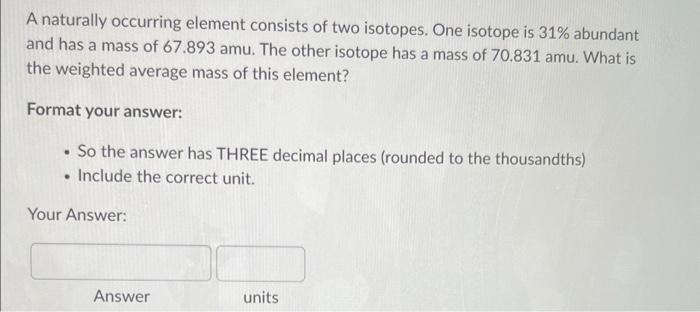
A naturally occurring element consists of two isotopes. One isotope is \( 31 \% \) abundant and has a mass of \( 67.893 \mathrm{amu} \). The other isotope has a mass of \( 70.831 \mathrm{amu} \). What is the weighted average mass of this element? Format your answer: - So the answer has THREE decimal places (rounded to the thousandths) - Include the correct unit. Your Answer: Answer units