Home /
Expert Answers /
Advanced Physics /
a-monoatomic-ideal-gas-is-taken-through-the-cycle-abca-shown-in-the-figure-express-all-the-pa490
(Solved): A monoatomic ideal gas is taken through the cycle ABCA shown in the figure. Express all the ...
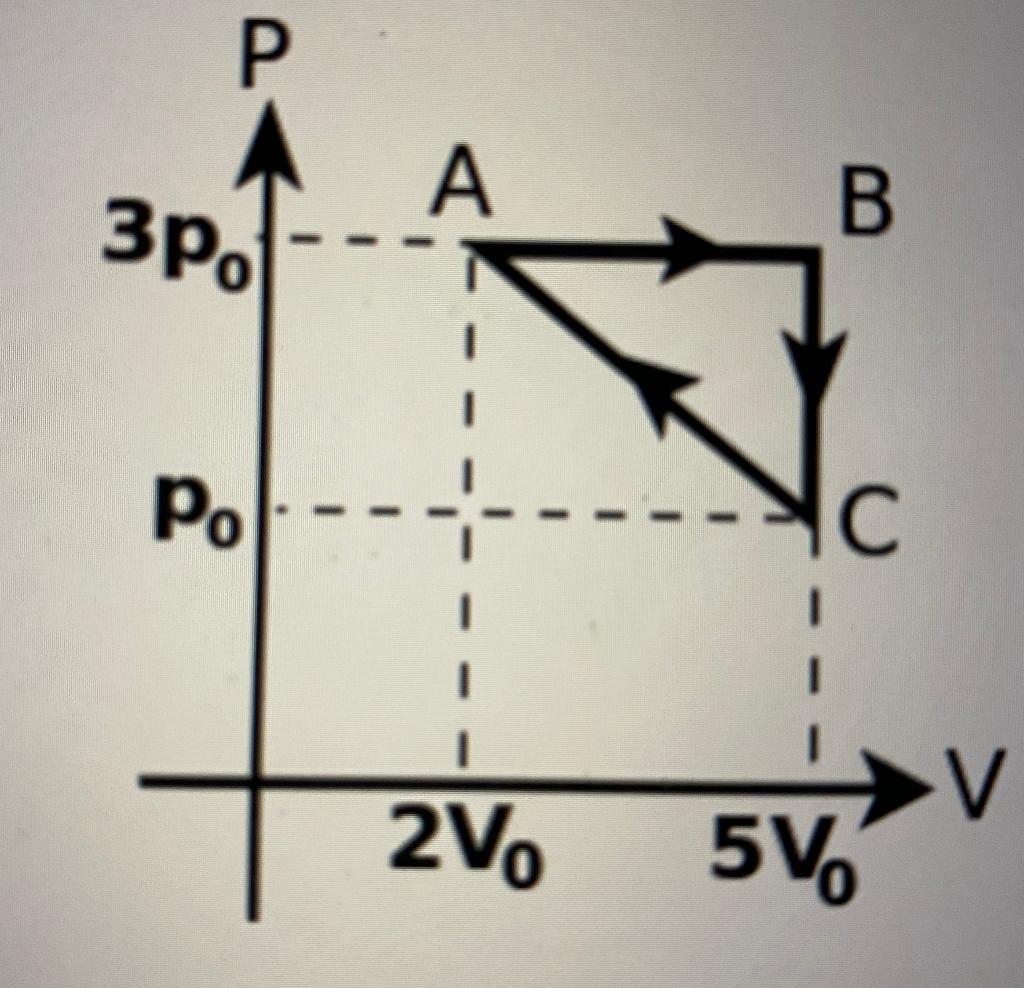
Part (a) Find the work WA?B done by the gas during process A ? B.
Part (b) Find the heat QA?B flowing into the gas during the process A ? B.
Part (c) Find the work WB?C done by the gas during the process B ? C.
Part (d) Find the heat QB?C flowing into the gas during the process B – C.
Part (e) Find the work done by the gas during process C ? A.
Part (f) Find the net work W done by the gas in the cycle.
Part (g) Find the heat QC?A flowing into the gas during the process C ? A.
Part (h) Find the net heat Q flowing into the gas in the complete cycle
Expert Answer
solution: A mono atomic ideal gas is taken through the cycle A?B?C?A shown in the question. n = 1 mole. firstly, internal energy: ?U=nCv?T=1×(32R)?T=1