Home /
Expert Answers /
Chemistry /
a-mixture-of-two-hydrocarbons-c8h18-octane-and-c7h8-toluene-has-a-mass-of-163g-pa550
(Solved): A mixture of two hydrocarbons, C8H18 (octane) and C7H8 (toluene), has a mass of 163g. ...
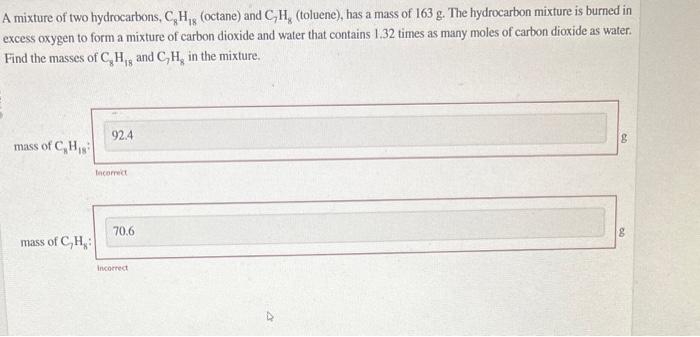
A mixture of two hydrocarbons, (octane) and (toluene), has a mass of . The hydrocarbon mixture is burned in excess oxygen to form a mixture of carbon dioxide and water that contains 1.32 times as many moles of carbon dioxide as water. Find the masses of and in the mixture. mass of bacerect g mass of