Home /
Expert Answers /
Chemistry /
a-mixture-of-mathbf-c-u-c-l-2-and-inert-material-is-analyzed-to-determine-the-cu-content-pa991
(Solved): A mixture of \( \mathbf{C u C l}_{2} \) and inert material is analyzed to determine the Cu content ...
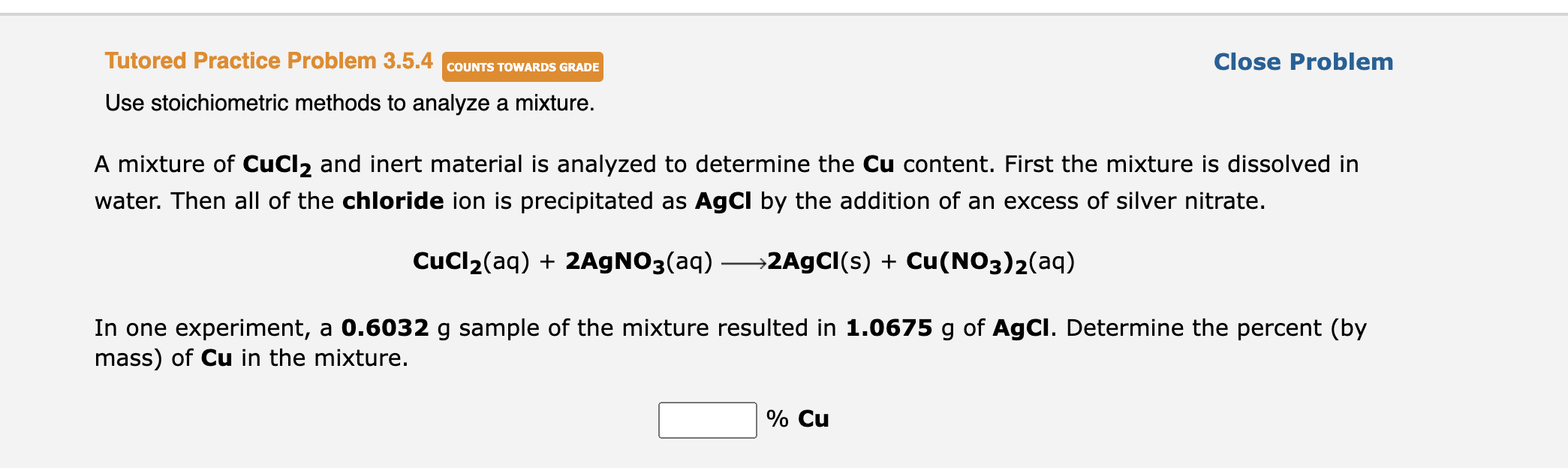
A mixture of \( \mathbf{C u C l}_{2} \) and inert material is analyzed to determine the Cu content. First the mixture is dissolved in water. Then all of the chloride ion is precipitated as \( \mathbf{A g C l} \) by the addition of an excess of silver nitrate. \[ \mathrm{CuCl}_{2}(\mathrm{aq})+2 \mathrm{AgNO}_{3}(\mathrm{aq}) \longrightarrow \mathbf{2 A g C l}(\mathrm{s})+\mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2}(\mathrm{aq}) \] In one experiment, a \( \mathbf{0 . 6 0 3 2} \mathrm{g} \) sample of the mixture resulted in \( \mathbf{1 . 0 6 7 5} \mathrm{g} \) of \( \mathbf{A g C l} \). Determine the percent (by mass) of \( \mathbf{C u} \) in the mixture.
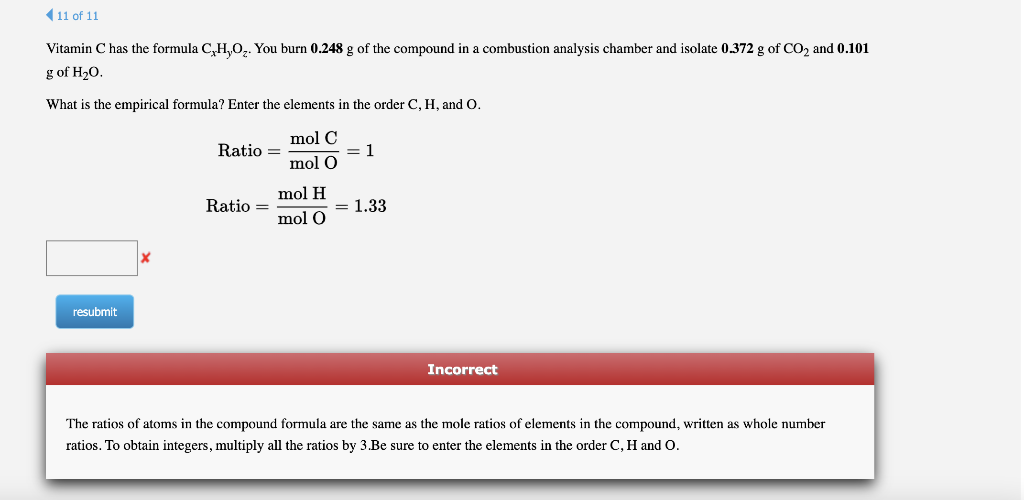
Vitamin \( \mathrm{C} \) has the formula \( \mathrm{C}_{x} \mathrm{H}_{y} \mathrm{O}_{z} \). You burn \( 0.248 \mathrm{~g} \) of the compound in a combustion analysis chamber and isolate \( 0.372 \mathrm{~g} \mathrm{of} \mathrm{CO}_{2} \) and \( 0.101 \) \( \mathrm{g} \) of \( \mathrm{H}_{2} \mathrm{O} \). What is the empirical formula? Enter the elements in the order \( \mathrm{C}, \mathrm{H} \), and \( \mathrm{O} \). \[ \begin{aligned} \text { Ratio } &=\frac{\operatorname{mol~C}}{\operatorname{mol~O}}=1 \\ \text { Ratio } &=\frac{\operatorname{mol~H}}{\mathrm{mol} \mathrm{O}}=1.33 \end{aligned} \] Incorrect The ratios of atoms in the compound formula are the same as the mole ratios of elements in the compound, written as whole number ratios. To obtain integers, multiply all the ratios by 3.Be sure to enter the elements in the order \( \mathrm{C}, \mathrm{H} \) and \( \mathrm{O} \).
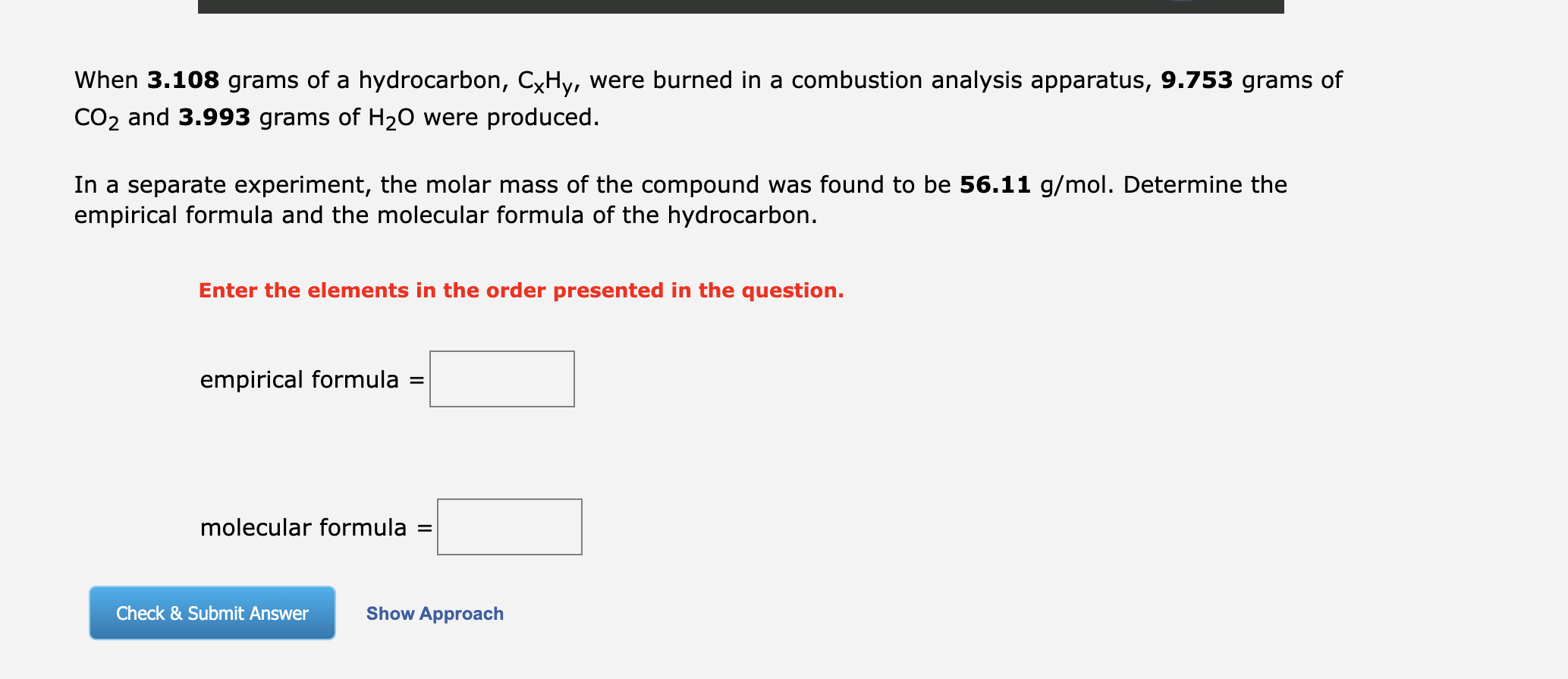
When \( 3.108 \) grams of a hydrocarbon, \( \mathrm{C}_{\mathrm{x}} \mathrm{H}_{\mathrm{y}} \), were burned in a combustion analysis apparatus, \( 9.753 \mathrm{grams} \) of \( \mathrm{CO}_{2} \) and \( \mathbf{3 . 9 9 3} \) grams of \( \mathrm{H}_{2} \mathrm{O} \) were produced. In a separate experiment, the molar mass of the compound was found to be \( 56.11 \mathrm{~g} / \mathrm{mol} \). Determine the empirical formula and the molecular formula of the hydrocarbon. Enter the elements in the order presented in the question. empirical formula \( = \) molecular formula \( = \)