Home /
Expert Answers /
Civil Engineering /
a-milliequivalent-per-litre-bar-graph-refer-to-the-figure-provided-and-the-hypothetical-combin-pa278
(Solved): A milliequivalent per litre bar graph (refer to the figure provided) and the hypothetical combin ...
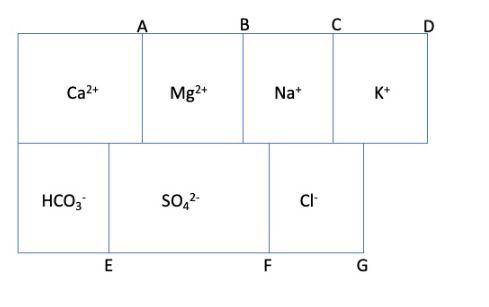
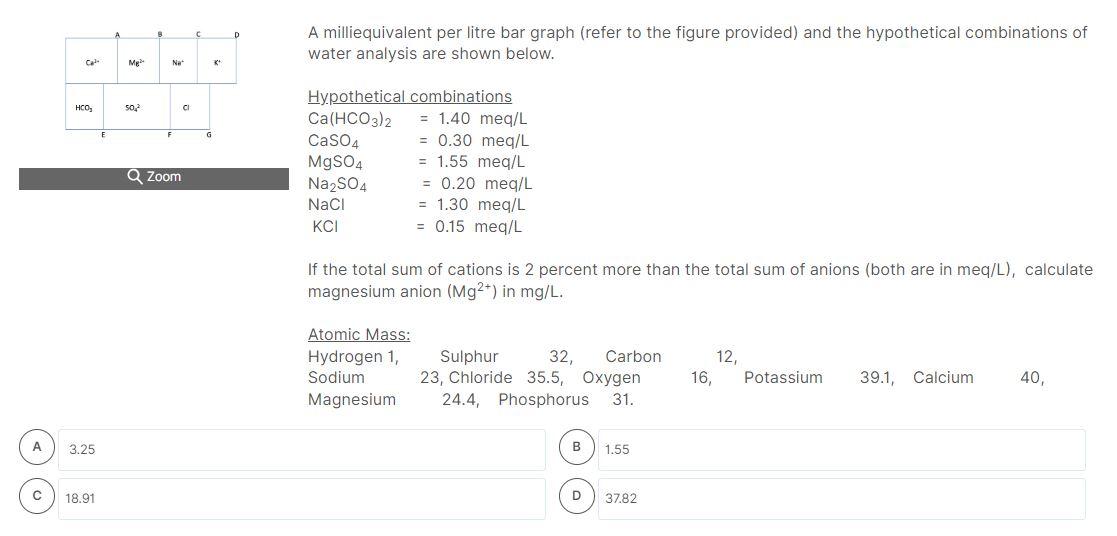
A milliequivalent per litre bar graph (refer to the figure provided) and the hypothetical combinations of water analysis are shown below. Hypothetical combinations \( \mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}=1.40 \mathrm{meq} / \mathrm{L} \) \( \mathrm{CaSO}_{4}=0.30 \mathrm{meq} / \mathrm{L} \) \( \mathrm{MgSO}_{4}=1.55 \mathrm{meq} / \mathrm{L} \) \( \mathrm{Na}_{2} \mathrm{SO}_{4}=0.20 \mathrm{meq} / \mathrm{L} \) \( \mathrm{NaCl} \quad=1.30 \mathrm{meq} / \mathrm{L} \) \( \mathrm{KCl}=0.15 \mathrm{meq} / \mathrm{L} \) If the total sum of cations is 2 percent more than the total sum of anions (both are in meq/L), calculate sulphate anion \( \left(\mathrm{SO}_{4}{ }^{2-}\right) \) in \( \mathrm{mg} / \mathrm{L} \). Atomic Mass: \( \begin{array}{lcccccc}\text { Hydrogen 1, } & \text { Sulphur } 32, \text { Carbon } & 12, & & \\ \text { Sodium } & \text { 23, Chloride } 35.5 \text {, Oxygen } & 16, & \text { Potassium } & \text { 39.1, Calcium } & \text { 40, }\end{array} \)
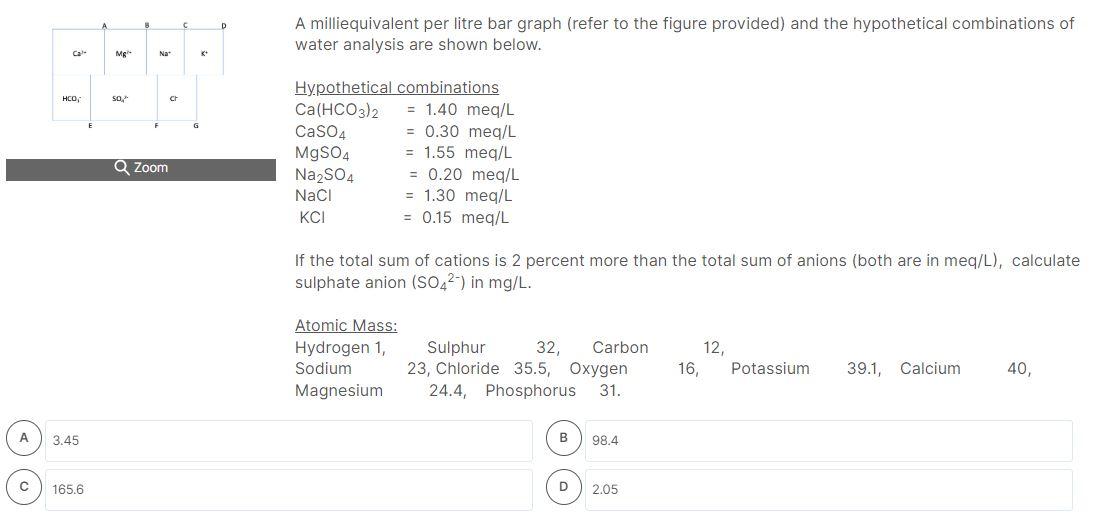
A milliequivalent per litre bar graph (refer to the figure provided) and the hypothetical combinations of water analysis are shown below. If the total sum of cations is 2 percent more than the total sum of anions (both are in meq/L), determine G. Atomic Mass: \( \begin{array}{lcccccc}\text { Hydrogen 1, } & \text { Sulphur } & 32, \text { Carbon } & 12, & & \\ \text { Sodium } & \text { 23, Chloride } 35.5, \text { Oxygen } & 16, & \text { Potassium } & 39.1, & \text { Calcium }\end{array} \) Magnesium \( 24.4 \), Phosphorus \( 31 . \) (B) D)
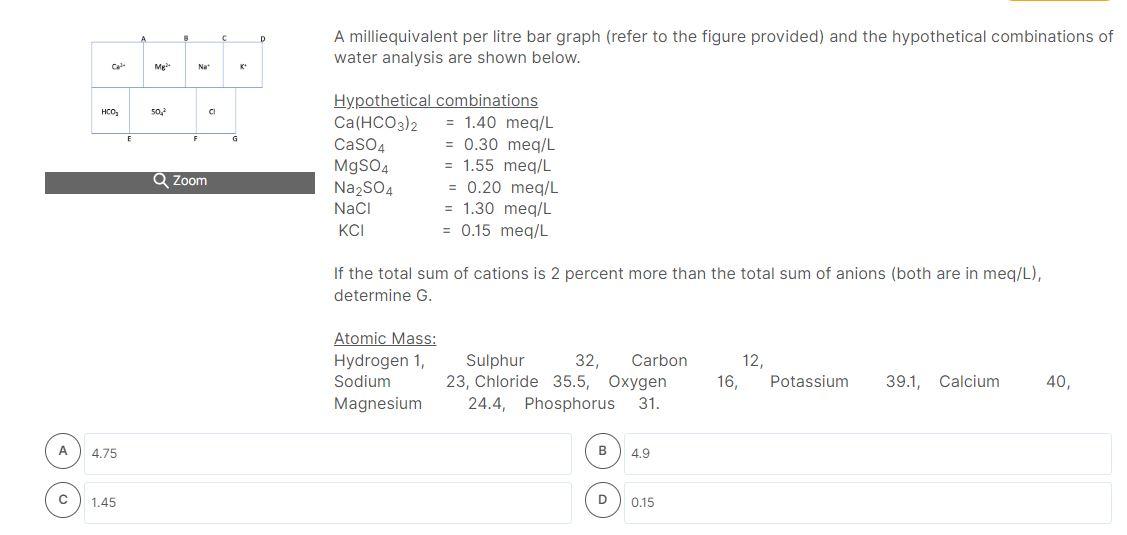
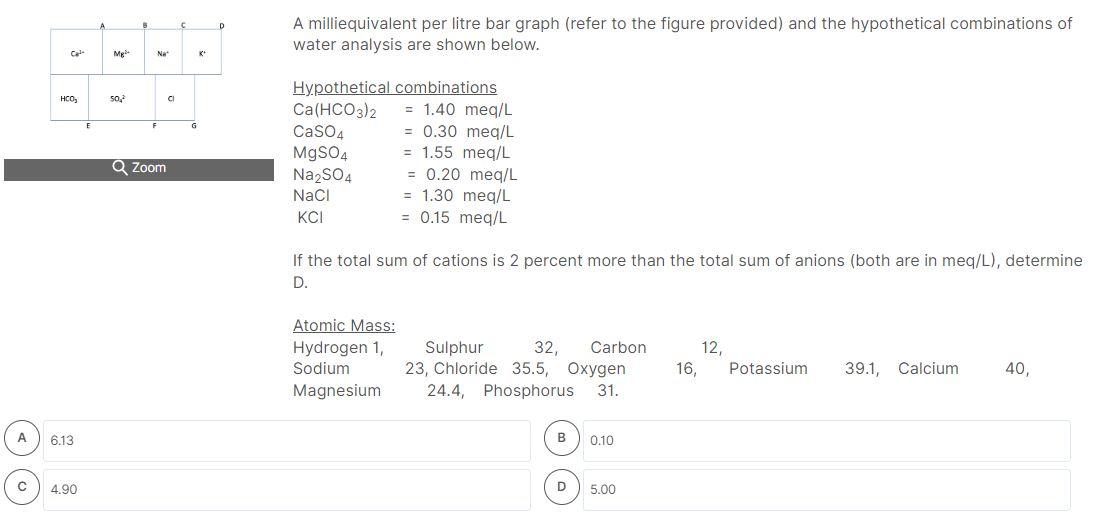
A milliequivalent per litre bar graph (refer to the figure provided) and the hypothetical combinations of water analysis are shown below. If the total sum of cations is 2 percent more than the total sum of anions (both are in meq/L), determine D. Atomic Mass: \( \begin{array}{lcccccc}\text { Hydrogen 1, } & \text { Sulphur } & 32, \text { Carbon } & 12, & & \\ \text { Sodium } & 23, \text { Chloride } & 35.5, \text { Oxygen } & 16, & \text { Potassium } & 39.1, \text { Calcium } & 40 \text {, }\end{array} \) Magnesium \( \quad 24.4 \), Phosphorus \( 31 . \)
A milliequivalent per litre bar graph (refer to the figure provided) and the hypothetical combinations of water analysis are shown below. If the total sum of cations is 2 percent more than the total sum of anions (both are in meq/L), calculate magnesium anion \( \left(\mathrm{Mg}^{2+}\right) \) in \( \mathrm{mg} / \mathrm{L} \). Atomic Mass: \( \begin{array}{lrrrrrr}\text { Hydrogen 1, } & \text { Sulphur } & 32, & \text { Carbon } & 12, & & \\ \text { Sodium } & 23, \text { Chloride } & 35.5, \text { Oxygen } & 16, & \text { Potassium } & 39.1, \text { Calcium } & 40,\end{array} \) Magnesium \( \quad 24.4 \), Phosphorus \( 31 . \) B
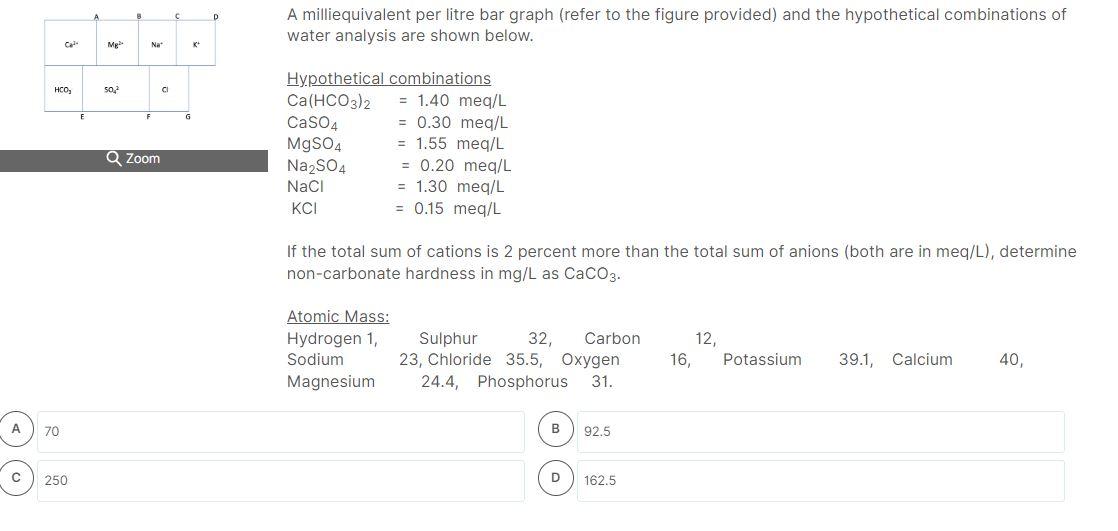
A milliequivalent per litre bar graph (refer to the figure provided) and the hypothetical combinations of water analysis are shown below. Hypothetical combinations \( \mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}=1.40 \mathrm{meq} / \mathrm{L} \) \( \mathrm{CaSO}_{4} \quad=0.30 \mathrm{meq} / \mathrm{L} \) \( \mathrm{MgSO}_{4}=1.55 \mathrm{meq} / \mathrm{L} \) \( \mathrm{Na}_{2} \mathrm{SO}_{4} \quad=0.20 \mathrm{meq} / \mathrm{L} \) \( \mathrm{NaCl}=1.30 \mathrm{meq} / \mathrm{L} \) \( \mathrm{KCl}=0.15 \mathrm{meq} / \mathrm{L} \) If the total sum of cations is 2 percent more than the total sum of anions (both are in meq/L), determine non-carbonate hardness in \( \mathrm{mg} / \mathrm{L} \) as \( \mathrm{CaCO}_{3} \). Atomic Mass: \begin{tabular}{lccccc} \hline Hydrogen 1, & Sulphur 32, Carbon & 12, & & \\ Sodium & 23, Chloride \( 35.5 \), Oxygen & 16, & Potassium & \( 39.1 \), Calcium & 40 , \end{tabular}