Home /
Expert Answers /
Chemistry /
a-mechanism-for-the-gas-phase-reaction-of-fluorine-with-chlorine-dioxide-that-is-consistent-with-th-pa667
(Solved): A mechanism for the gas phase reaction of fluorine with chlorine dioxide that is consistent with th ...
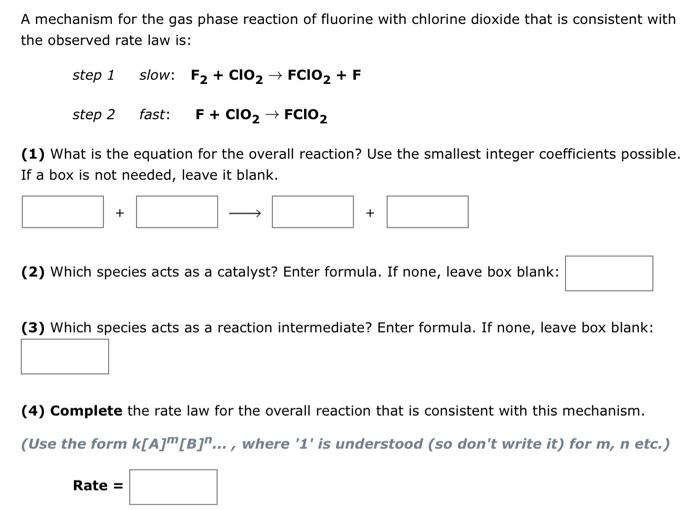
A mechanism for the gas phase reaction of fluorine with chlorine dioxide that is consistent with the observed rate law is: step 1 slow: \( \mathbf{F}_{2}+\mathbf{C l O}_{2} \rightarrow \mathbf{F C l O}_{2}+\mathbf{F} \) step 2 fast: \( \mathbf{F}+\mathbf{C l O}_{2} \rightarrow \mathbf{F C I O}_{2} \) (1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank. (2) Which species acts as a catalyst? Enter formula. If none, leave box blank: (3) Which species acts as a reaction intermediate? Enter formula. If none, leave box blank: (4) Complete the rate law for the overall reaction that is consistent with this mechanism. (Use the form \( k[A]^{m}[B]^{n} \ldots \), where ' 1 ' is understood (so don't write it) for \( m, n \) etc.)