Home /
Expert Answers /
Chemistry /
a-identify-and-write-oxidation-and-reduction-half-cell-reactions-for-the-following-equation-2-pa933
(Solved): (a) Identify and write oxidation and reduction half-cell reactions for the following equation. (2 ...
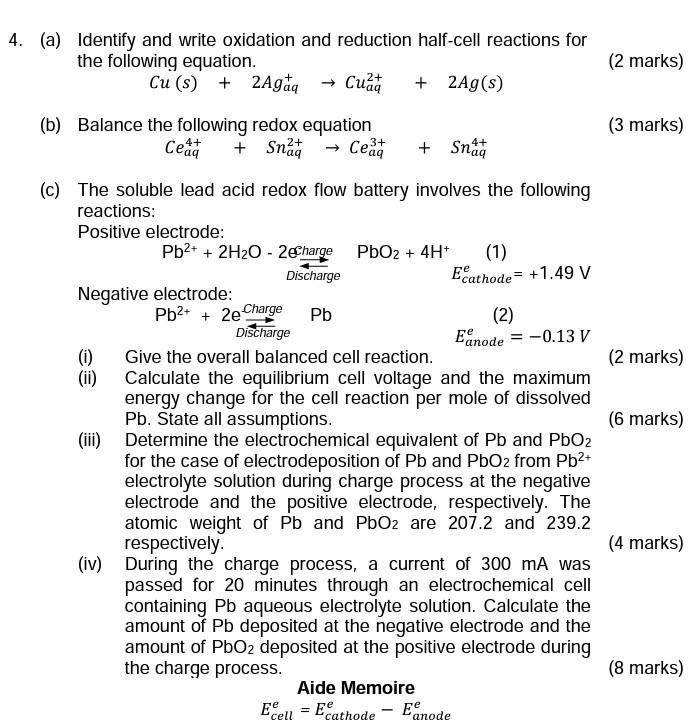
(a) Identify and write oxidation and reduction half-cell reactions for the following equation. (2 marks) \[ C u(s)+2 A g_{a q}^{+} \rightarrow C u_{a q}^{2+}+2 A g(s) \] (b) Balance the following redox equation (3 marks) \[ \mathrm{Ce}_{a q}^{4+}+\mathrm{Sn}_{a q}^{2+} \rightarrow \mathrm{Ce}_{a q}^{3+}+\mathrm{Sn}_{a q}^{4+} \] (c) The soluble lead acid redox flow battery involves the following reactions: Positive electrode: Negative electrode: \[ \mathrm{Pb}^{2+}+2 \underset{\text { Discharge }}{\stackrel{\text { Charge }}{\rightleftarrows}} \mathrm{Pb} \quad E_{\text {anode }}^{e}=-0.13 \mathrm{~V} \] (i) Give the overall balanced cell reaction. (2 marks) energy change for the cell reaction per mole of dissolved \( \mathrm{Pb} \). State all assumptions. (6 marks) (iii) Determine the electrochemical equivalent of \( \mathrm{Pb} \) and \( \mathrm{PbO}_{2} \) for the case of electrodeposition of \( \mathrm{Pb} \) and \( \mathrm{PbO}_{2} \) from \( \mathrm{Pb}^{2+} \) electrolyte solution during charge process at the negative electrode and the positive electrode, respectively. The atomic weight of \( \mathrm{Pb} \) and \( \mathrm{PbO}_{2} \) are \( 207.2 \) and \( 239.2 \) respectively. (4 marks) (iv) During the charge process, a current of \( 300 \mathrm{~mA} \) was passed for 20 minutes through an electrochemical cell containing \( \mathrm{Pb} \) aqueous electrolyte solution. Calculate the amount of \( \mathrm{Pb} \) deposited at the negative electrode and the amount of \( \mathrm{PbO}_{2} \) deposited at the positive electrode during the charge process. (8 marks) Aide Memoire \[ E_{\text {cell }}^{e}=E_{\text {cathode }}^{e}-E_{\text {anode }}^{e} \]