Home /
Expert Answers /
Chemistry /
a-galvanic-cell-using-cut-cu-and-zn-zn-was-set-up-at-369-k-b-determine-delta-g-for-the-cellc-us-pa292
(Solved): A galvanic cell using Cut/Cu and Zn+/Zn was set up at 369 K.b) determine delta G for the cellc) us ...
A galvanic cell using Cut/Cu and Zn²+/Zn was set up at 369 K.
b) determine delta G for the cell
c) using the Nernst equation and rhe values calculated above and provided in this question, determine the value of Q when E = 1.1597 V at 369K
I understand how to complete a) but need some help with b) and c). thanks!
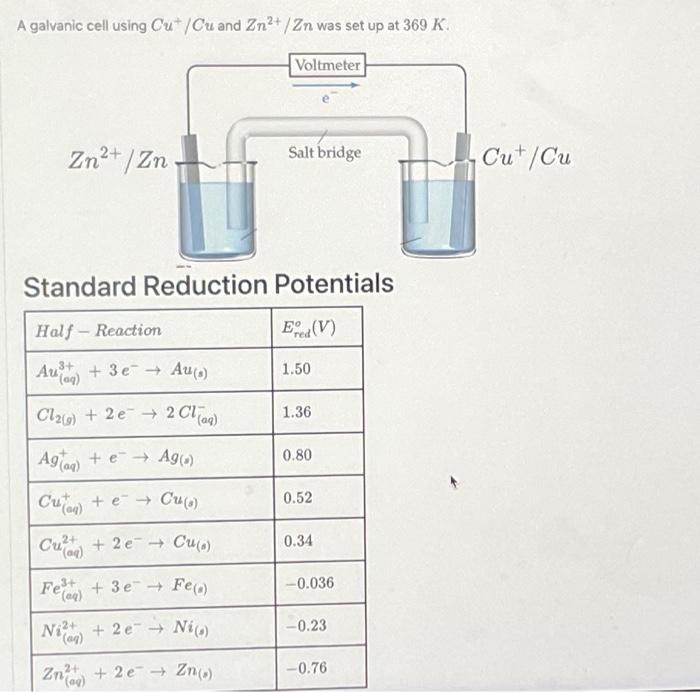


A galvanic cell using and was set up at . Standard Reduction Potentials
a) Determine the standard potential of the cell: b) Determine for this cell: c) Using the Nernst Equation and the values calculated above and provided in this question, determine the value of when at : Equations and Constants