Home /
Expert Answers /
Chemistry /
a-galvanic-cell-using-cu-cu-and-zn2-zn-was-set-up-at-337k-and-the-non-standard-cell-potential-pa956
(Solved): A galvanic cell using Cu+/Cu and Zn2+/Zn was set up at 337K and the non-standard cell potential ...
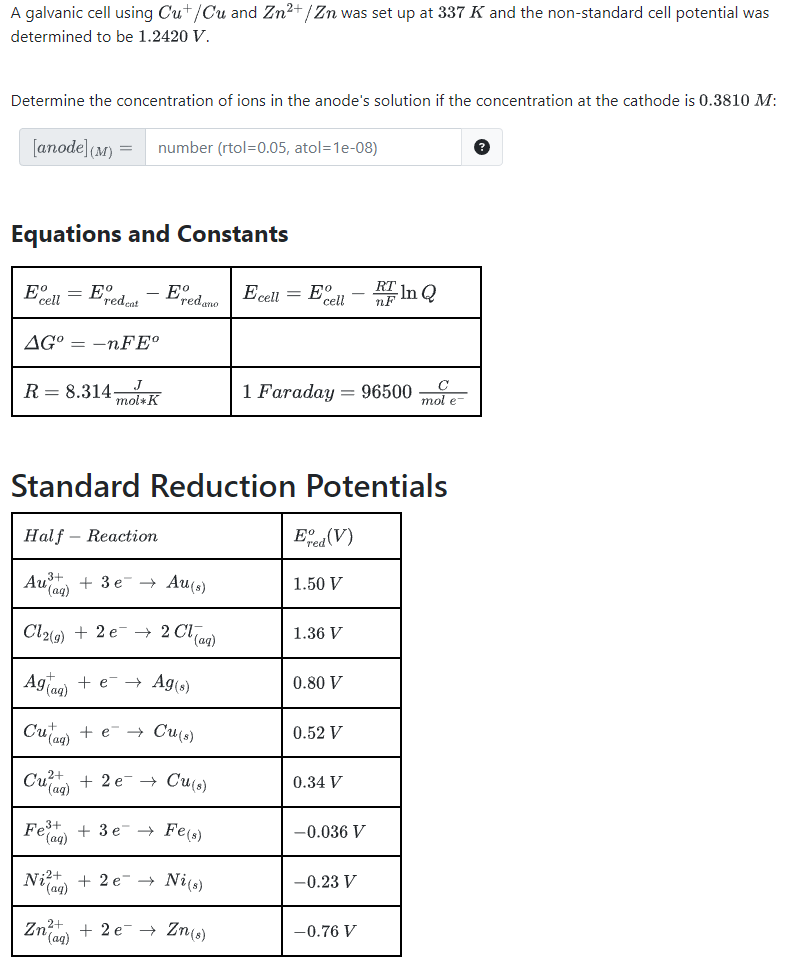
A galvanic cell using and was set up at and the non-standard cell potential was determined to be . Determine the concentration of ions in the anode's solution if the concentration at the cathode is : number rtol , atol Equations and Constants Standard Reduction Potentials