Home /
Expert Answers /
Chemistry /
a-galvanic-cell-is-powered-by-the-following-redox-reaction-2-mathrm-mno-4-a-q-4-mathr-pa644
(Solved): A galvanic cell is powered by the following redox reaction: \[ 2 \mathrm{MnO}_{4}^{-}(a q)+4 \mathr ...
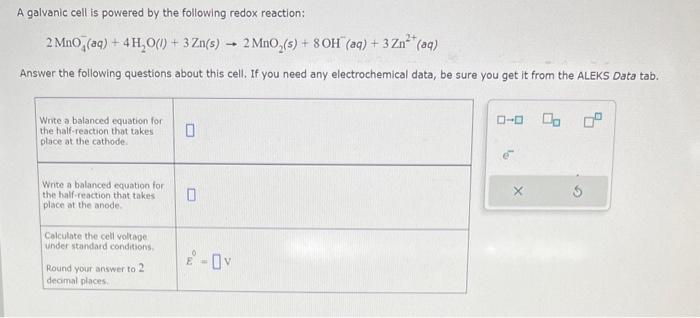
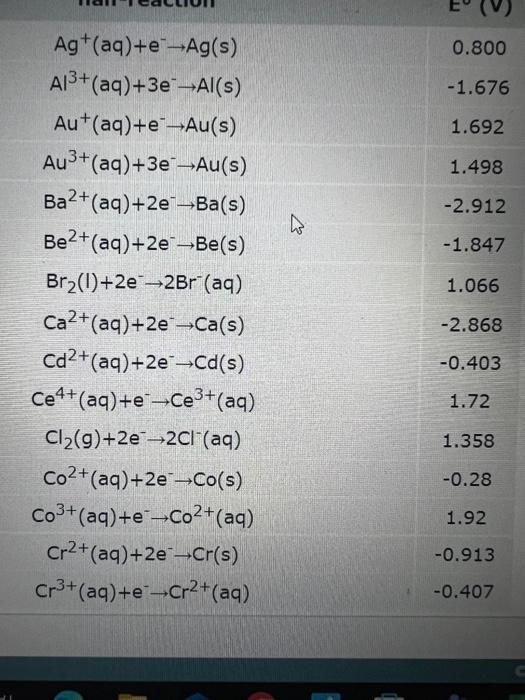



A galvanic cell is powered by the following redox reaction: \[ 2 \mathrm{MnO}_{4}^{-}(a q)+4 \mathrm{H}_{2} \mathrm{O}(l)+3 \mathrm{Zn}(s) \rightarrow 2 \mathrm{MnO}_{2}(s)+8 \mathrm{OH}^{-}(a q)+3 \mathrm{Zn}^{2+}(a q) \] Answer the following questions about this cell. If you need any electrochemical data, b
\( \begin{array}{lc}\mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ag}(\mathrm{s}) & 0.800 \\ \mathrm{Al}^{3+}(\mathrm{aq})+3 \mathrm{e}^{-} \rightarrow \mathrm{Al}(\mathrm{s}) & -1.676 \\ \mathrm{Au}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Au}(\mathrm{s}) & 1.692 \\ \mathrm{Au}^{3+}(\mathrm{aq})+3 \mathrm{e}^{-} \rightarrow \mathrm{Au}(\mathrm{s}) & 1.498 \\ \mathrm{Ba}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Ba}(\mathrm{s}) & -2.912 \\ \mathrm{Be}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Be}(\mathrm{s}) & -1.847 \\ \mathrm{Br}_{2}(\mathrm{I})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Br}^{-}(\mathrm{aq}) & 1.066 \\ \mathrm{Ca}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Ca}(\mathrm{s}) & -2.868 \\ \mathrm{Cd}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Cd}(\mathrm{s}) & -0.403 \\ \mathrm{Ce}^{4+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ce}^{3+}(\mathrm{aq}) & 1.72 \\ \mathrm{Cl}_{2}(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cl}^{-}(\mathrm{aq}) & 1.358 \\ \mathrm{Co}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Co}(\mathrm{s}) & -0.28 \\ \mathrm{Co}^{3+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Co}^{2+}(\mathrm{aq}) & 1.92 \\ \mathrm{Cr}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Cr}(\mathrm{s}) & -0.913 \\ \mathrm{Cr}^{3+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Cr}^{2+}(\mathrm{aq}) & -0.407\end{array} \)
\( \mathrm{Cr}^{3+}(\mathrm{aq})+3 \mathrm{e}^{-} \rightarrow \mathrm{Cr}(\mathrm{s}) \quad-0.744 \) \( \mathrm{CrO}_{4}{ }^{2-}(\mathrm{aq})+4 \mathrm{H}_{2} \mathrm{O}(\mathrm{I})+3 \mathrm{e}^{-} \rightarrow \mathrm{Cr}(\mathrm{OH})_{3}(\mathrm{~s})+5 \mathrm{OH}^{-}(\mathrm{aq}) \quad-0.13 \) \( \mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}(\mathrm{aq})+14 \mathrm{H}^{+}(\mathrm{aq})+6 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cr}^{3+}(\mathrm{aq})+7 \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \quad 1.36 \) \( \mathrm{Cu}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Cu}(\mathrm{s}) \quad 0.521 \) \( \mathrm{Cu}^{2+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Cu}^{+}(\mathrm{aq}) \quad 0.153 \) \( \mathrm{Cu}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Cu}(\mathrm{s}) \quad 0.342 \) \( \mathrm{~F}_{2}(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{~F}^{-}(\mathrm{aq}) \quad 2.866 \) \( \mathrm{Fe}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Fe}(\mathrm{s}) \) \( \mathrm{Fe}^{3+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Fe}^{2+}(\mathrm{aq}) \quad 0.771 \) \( \mathrm{Fe}^{3+}(\mathrm{aq})+3 \mathrm{e}^{-} \rightarrow \mathrm{Fe}(\mathrm{s}) \quad-0.037 \) \( 2 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2}(\mathrm{~g}) \quad 0.000 \) \( 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{I})+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2}(\mathrm{~g})+2 \mathrm{OH}^{-}(\mathrm{aq}) \quad-0.828 \) \( \mathrm{H}_{2} \mathrm{O}_{2}(\mathrm{aq})+2 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \quad 1.776 \) \( \mathrm{Hg}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Hg}(\mathrm{I}) \quad 0.851 \) \( 2 \mathrm{Hg}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Hg}_{2}^{2+}(\mathrm{aq}) \quad 0.92 \) \( \mathrm{Hg}_{2}{ }^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Hg}(\mathrm{I}) \) \( \mathrm{I}_{2}(\mathrm{~s})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{I}^{-}(\mathrm{aq}) \)
\( 2 \mathrm{IO}_{3}{ }^{-}(\mathrm{aq})+12 \mathrm{H}^{+}(\mathrm{aq})+10 \mathrm{e}^{-} \rightarrow \mathrm{I}_{2}(\mathrm{~s})+6 \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \quad 1.195 \) \( \mathrm{~K}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{K}(\mathrm{s}) \quad-2.931 \) \( \mathrm{Li}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Li}(\mathrm{s}) \quad-3.040 \) \( \mathrm{Mg}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Mg}(\mathrm{s}) \quad-2.372 \) \( \mathrm{Mn}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Mn}(\mathrm{s}) \quad-1.185 \) \( \mathrm{MnO}_{2}(\mathrm{~s})+4 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}(\mathrm{aq})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \quad 1.224 \) \( \mathrm{MnO}_{4}^{-}(\mathrm{aq})+8 \mathrm{H}^{+}(\mathrm{aq})+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}(\mathrm{aq})+4 \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \quad 1.507 \) \( \mathrm{MnO}_{4}^{-}(\mathrm{aq})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{I})+3 \mathrm{e}^{-} \rightarrow \mathrm{MnO}_{2}(\mathrm{~s})+4 \mathrm{OH}^{-}(\mathrm{aq}) \quad 0.595 \) \( \mathrm{HNO}_{2}(\mathrm{aq})+\mathrm{H}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{NO}(\mathrm{g})+\mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \quad 0.983 \) \( \mathrm{~N}_{2}(\mathrm{~g})+5 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \rightarrow \mathrm{N}_{2} \mathrm{H}_{5}^{+}(\mathrm{aq}) \quad-0.214 \) \( \mathrm{~N}_{2}(\mathrm{~g})+4 \mathrm{H}_{2} \mathrm{O}(\mathrm{I})+4 \mathrm{e}^{-} \rightarrow 4 \mathrm{OH}^{-}(\mathrm{aq})+\mathrm{N}_{2} \mathrm{H}_{4}(\mathrm{aq}) \quad-1.16 \) \( \mathrm{NO}_{3}^{-}(\mathrm{aq})+4 \mathrm{H}^{+}(\mathrm{aq})+3 \mathrm{e}^{-} \rightarrow \mathrm{NO}(\mathrm{g})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \quad 0.957 \) \( \mathrm{Na}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Na}(\mathrm{s}) \quad-2.71 \) \( \mathrm{Ni}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Ni}(\mathrm{s}) \quad-0.257 \) \( \mathrm{O}_{2}(\mathrm{~g})+2 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_{2} \mathrm{O}_{2}(\mathrm{aq}) \quad 0.695 \) \( \mathrm{O}_{2}(\mathrm{~g})+4 \mathrm{H}^{+}(\mathrm{aq})+4 \mathrm{e}^{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \) \( \mathrm{O}_{2}(\mathrm{~g})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{I})+4 \mathrm{e}^{-} \rightarrow 4 \mathrm{OH}^{-}(\mathrm{aq}) \quad 0.401 \) \( \mathrm{O}_{2}(\mathrm{a})+2 \mathrm{H}^{+}(\mathrm{aa})+2 \mathrm{e}^{-} \rightarrow \mathrm{O}_{2}(\mathrm{a})+\mathrm{H}_{2} \mathrm{O}(1) \quad 2.076 \)
acids at \( 25^{\circ} \mathrm{C} \)