Home /
Expert Answers /
Chemistry /
a-galvanic-cell-consists-of-one-half-cell-that-contains-mathrm-pt-s-and-mathrm-pt-2-pa840
(Solved): A galvanic cell consists of one half-cell that contains \( \mathrm{Pt}(s) \) and \( \mathrm{Pt}^{2+ ...
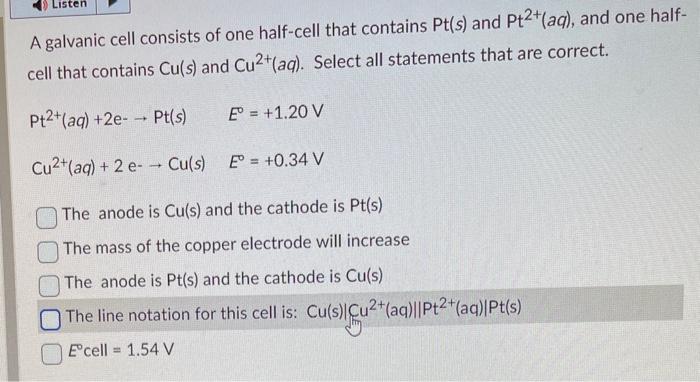
A galvanic cell consists of one half-cell that contains \( \mathrm{Pt}(s) \) and \( \mathrm{Pt}^{2+}(a q) \), and one halfcell that contains \( \mathrm{Cu}(s) \) and \( \mathrm{Cu}^{2+}(a q) \). Select all statements that are correct. \[ \begin{array}{ll} \mathrm{Pt}^{2+}(a q)+2 \mathrm{e}-\rightarrow \mathrm{Pt}(s) & E^{\circ}=+1.20 \mathrm{~V} \\ \mathrm{Cu}^{2+}(a q)+2 \mathrm{e}-\rightarrow \mathrm{Cu}(s) & E^{\circ}=+0.34 \mathrm{~V} \end{array} \] The anode is \( \mathrm{Cu}(\mathrm{s}) \) and the cathode is \( \mathrm{Pt}(\mathrm{s}) \) The mass of the copper electrode will increase The anode is \( \mathrm{Pt}(\mathrm{s}) \) and the cathode is \( \mathrm{Cu}(\mathrm{s}) \) The line notation for this cell is: \( \mathrm{Cu}(\mathrm{s})\left|\mathrm{Cu}^{2+}(\mathrm{aq}) \| \mathrm{Pt}^{2+}(\mathrm{aq})\right| \mathrm{Pt}(\mathrm{s}) \) \( E^{\circ} \) cell \( =1.54 \mathrm{~V} \)