Home /
Expert Answers /
Chemistry /
a-free-radical-reaction-is-a-chemical-reaction-involving-free-radicals-as-intermediates-the-stabil-pa250
(Solved): A free-radical reaction is a chemical reaction involving free radicals as intermediates. The stabil ...
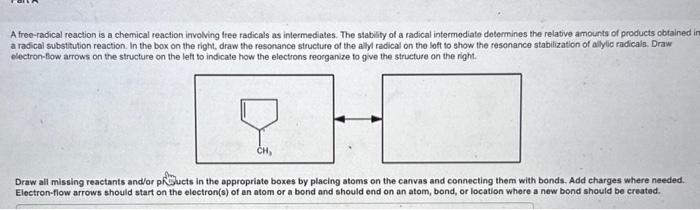
A free-radical reaction is a chemical reaction involving free radicals as intermediates. The stability of a radical intermediate determines the relative amounts of products cotained a radical substitution reaction. In the box on the right, draw the rescnance structure of the aly radical on the loft to show the resonance stabilization of alylic radicals. Draw electron-llow arrows on the structure on the left to indicate how the electrons reorganize to give the structure on the right. Draw all missing reactants and/or piflucts in the appropriate boxes by placing atoms on the canvas and connecting them with bonds, Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created.