Home /
Expert Answers /
Chemistry /
a-fill-in-any-missing-hydrogens-and-lone-pair-electrons-in-the-structure-of-anion-3-below-and-pa240
(Solved): (a) Fill in any missing hydrogens and lone pair electrons in the structure of anion 3 (below), and ...
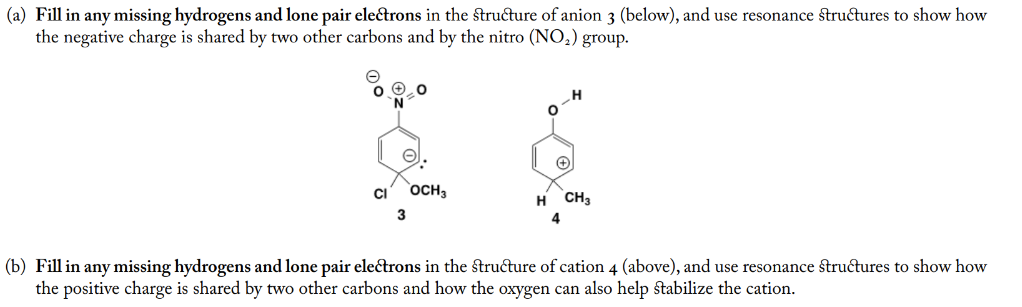
(a) Fill in any missing hydrogens and lone pair electrons in the structure of anion 3 (below), and use resonance structures to show how the negative charge is shared by two other carbons and by the nitro group. (b) Fill in any missing hydrogens and lone pair electrons in the structure of cation 4 (above), and use resonance structures to show how the positive charge is shared by two other carbons and how the oxygen can also help stabilize the cation.
Expert Answer
a.) The given anion has a negative charge which is in the conjugation with group (nitro).