Home /
Expert Answers /
Physics /
a-diatomic-ideal-gas-goes-through-the-cycle-a-b-c-d-a-as-shown-in-the-figure-proces-pa389
(Solved): A diatomic ideal gas goes through the cycle a b c d a as shown in the figure. Proces ...
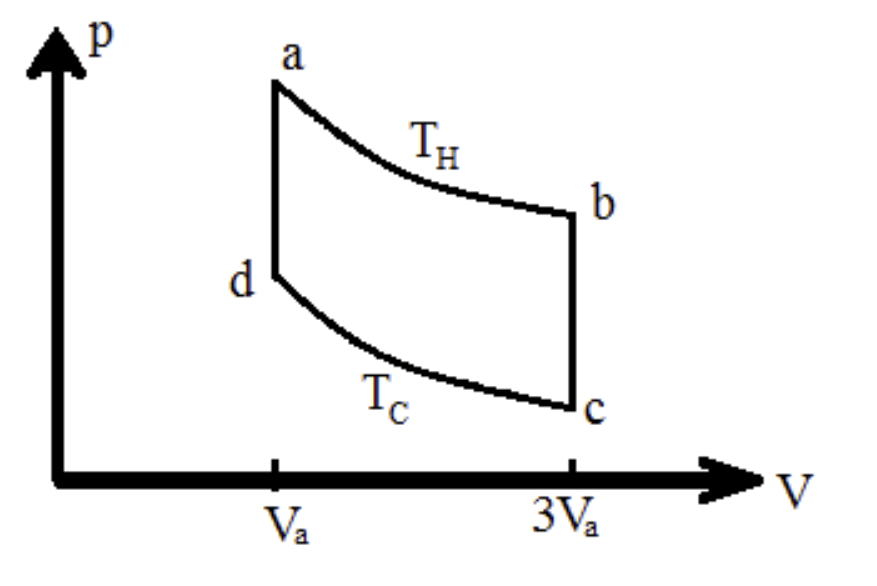
A diatomic ideal gas goes through the cycle a ? b ? c ? d ? a as shown in the figure. Processes ab and cd are isothermal and occur at temperatures TH = 390 K and TC = 295 K, respectively. There are n = 35 moles of this gas in the system, and the initial volume is Va = 0.0035 m3.
Part (a) Calculate the work Wab, in joules, done by the gas during the process a ? b.
Part (b) Calculate the work Wbc done by the gas, in joules, during the process b ? c.
Part (c) Calculate the total work W done in the entire cycle, in joules.
Part (d) Calculate the total heat Q, in joules, flowing into the gas in a complete cycle.