Home /
Expert Answers /
Chemistry /
a-determination-of-the-acetic-acid-in-vinegar-vinegar-is-a-solution-of-acetic-acid-pa960
(Solved): A. Determination of the \( \% \) acetic acid in vinegar Vinegar is a solution of acetic acid, \( \ ...
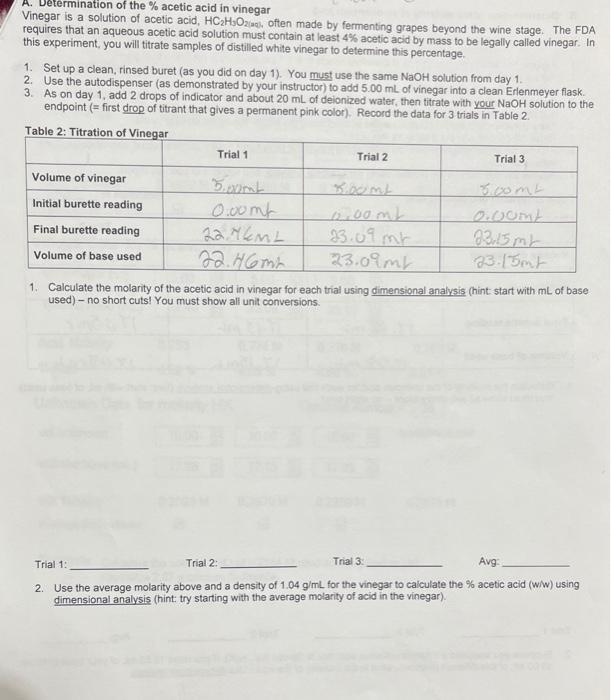
A. Determination of the \( \% \) acetic acid in vinegar Vinegar is a solution of acetic acid, \( \mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}\left(\mathrm{ac}_{1}\right. \), often made by fermenting grapes beyond the wine stage. The FDA requires that an aqueous acetic acid solution must contain at least \( 4 \% \) acetic acid by mass to be legally called vinegar. In this experiment, you will titrate samples of distilled white vinegar to determine this percentage. 1. Set up a clean, rinsed buret (as you did on day 1). You must use the same \( \mathrm{NaOH} \) solution from day 1. 2. Use the autodispenser (as demonstrated by your instructor) to add \( 5.00 \mathrm{~mL} \) of vinegar into a clean Erlenmeyer flask. 3. As on day 1, add 2 drops of indicator and about \( 20 \mathrm{~mL} \) of deionized water, then titrate with your \( \mathrm{NaOH} \) solution to the endpoint (= first drop of titrant that gives a permanent pink color). Record the data for 3 trials in Table 2. 1. Calculate the molarity of the acetic acid in vinegar for each trial using dimensional analysis (hint start with \( \mathrm{mL} \) of base used) - no short cuts! You must show all unit conversions. Trial 1: Trial 2: Trial 3 : Avg: 2. Use the average molarity above and a density of \( 1.04 \mathrm{~g} / \mathrm{mL} \) for the vinegar to calculate the \( \% \) acetic acid (W/w) using dimensional analysis (hint: try starting with the average molarity of acid in the vinegar).
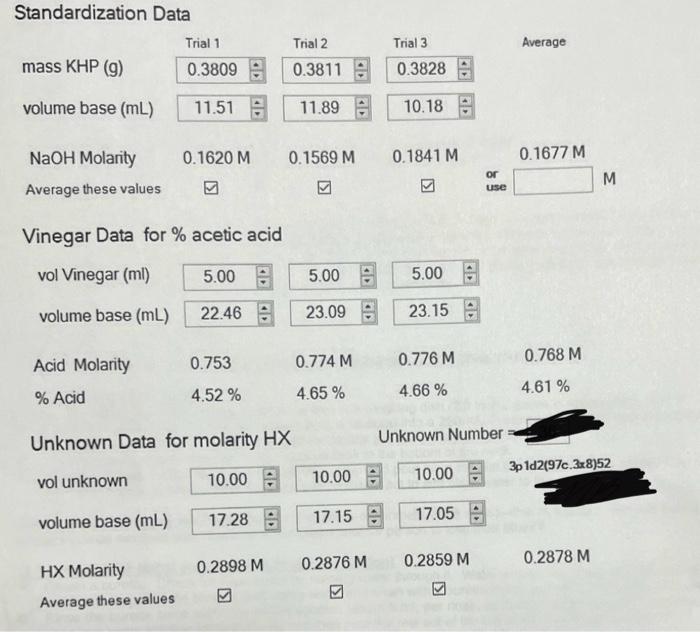
Standardization Data mass KHP \( (\mathrm{g}) \) \begin{tabular}{l} Trial 1 \\ \hline \( 0.3809 \) \\ \hline \end{tabular} \begin{tabular}{l} Trial 2 \\ \( 0.3811 \) \\ \hline \end{tabular} Trial 3 Average volume base \( (\mathrm{mL}) \) \( 11.51 \) \( 11.89 \) \( 10.18 \) : Vinegar Data for \( \% \) acetic acid vol Vinegar (ml) \begin{tabular}{rr|} \( 5.00 \div 5.00 \) \\ \( 22.46 \div 53.09 \) \\ \hline \end{tabular} \( 5.00 \) volume base \( (\mathrm{mL}) \) \( 23.15 \) \( \begin{array}{llllc}\text { Acid Molarity } & 0.753 & 0.774 \mathrm{M} & 0.776 \mathrm{M} & 0.768 \mathrm{M} \\ \text { \% Acid } & 4.52 \% & 4.65 \% & 4.66 \% & 4.61 \%\end{array} \) Unknown Data for molarity \( \mathrm{HX} \) Unknown Number vol unknown \( 10.00 \) \( 10.00 \) \( \begin{array}{rr}10.00 \div \\ 17.05 & \div\end{array} \) 3p 1d2(97c. 3x.8)52 volume base \( (\mathrm{mL}) \) HX Molarity \( \begin{array}{llll}0.2898 \mathrm{M} & 0.2876 \mathrm{M} & 0.2859 \mathrm{M} & 0.2878 \mathrm{M}\end{array} \) Average these values
Expert Answer
Molarity of NaOH = 0.1677 M (M1V1)acid = (M2V2)b