Home /
Expert Answers /
Chemical Engineering /
a-derive-an-equation-relating-the-joule-thomson-coefficient-jt-to-the-volume-expansion-coe-pa307
(Solved): (a) Derive an equation relating the Joule-Thomson coefficient (JT) to the volume expansion coe ...
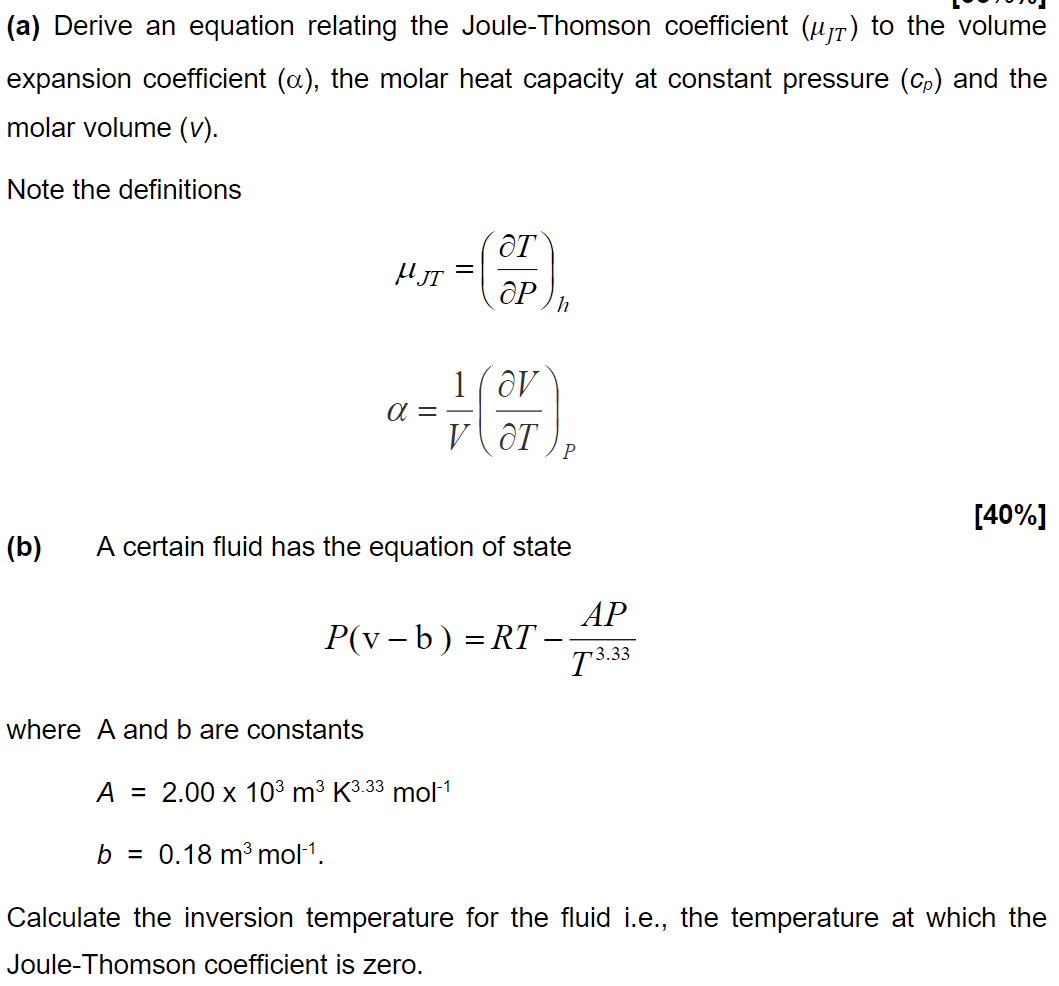
(a) Derive an equation relating the Joule-Thomson coefficient to the volume expansion coefficient , the molar heat capacity at constant pressure and the molar volume . Note the definitions (b) A certain fluid has the equation of state where and are constants Calculate the inversion temperature for the fluid i.e., the temperature at which the Joule-Thomson coefficient is zero.