Home /
Expert Answers /
Chemistry /
a-convert-the-below-amino-acid-fischer-projection-to-a-tetrahedral-structure-using-the-4-carbon-pa759
(Solved): (a) Convert the below amino acid (Fischer Projection) to a tetrahedral structure (using the 4-carbon ...
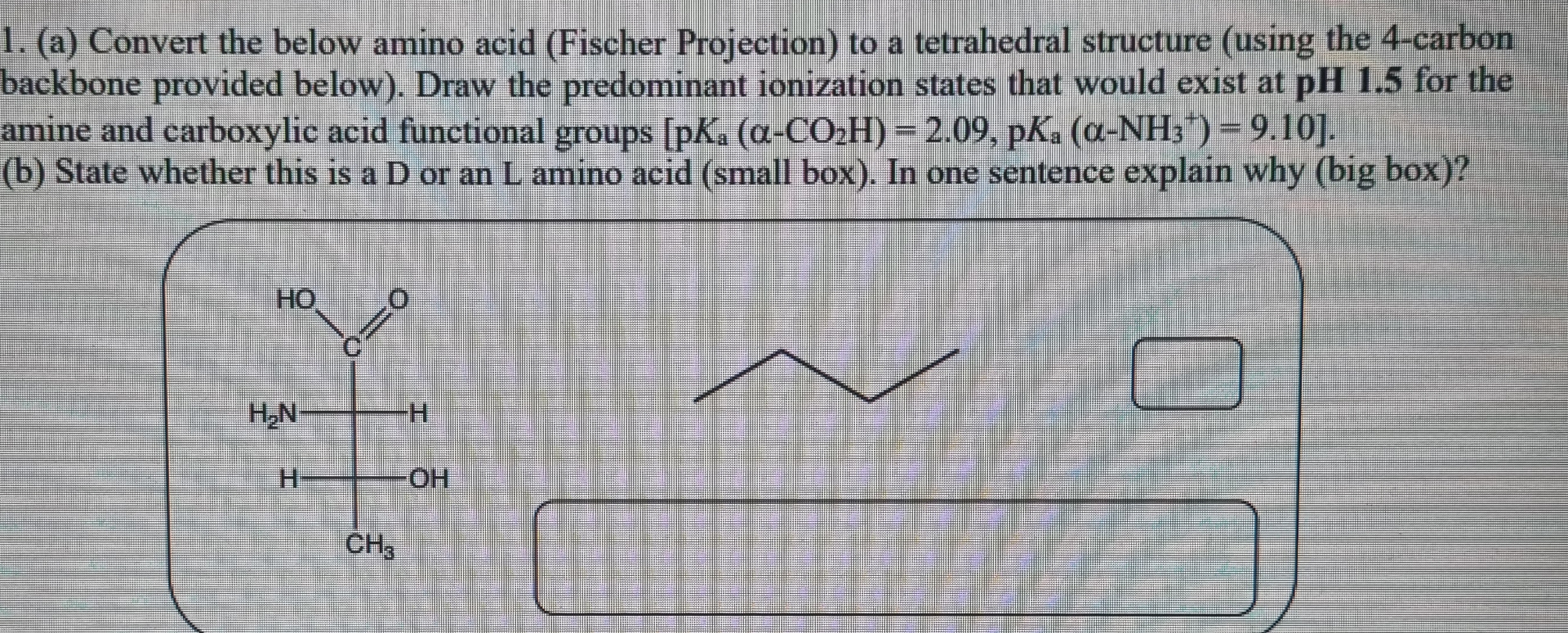
(a) Convert the below amino acid (Fischer Projection) to a tetrahedral structure (using the 4-carbon backbone provided below). Draw the predominant ionization states that would exist at
pH1.5for the amine and carboxylic acid functional groups
pK_(a)(\alpha -CO_(2)H)=2.09,pK_(a)(\alpha -NH_(3)^(+))=9.10. (b) State whether this is a D or an
Lamino acid (small box). In one sentence explain why (big box)?