Home /
Expert Answers /
Chemistry /
a-concentration-cell-similar-to-the-one-shown-is-composed-of-two-zn-electrodes-and-solutions-of-dif-pa518
(Solved): A concentration cell similar to the one shown is composed of two Zn electrodes and solutions of dif ...
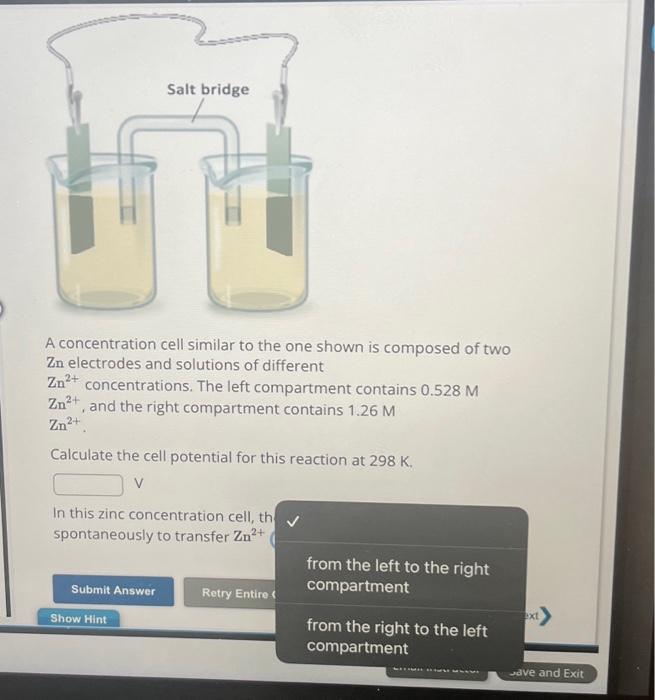

A concentration cell similar to the one shown is composed of two electrodes and solutions of different concentrations. The left compartment contains , and the right compartment contains . Calculate the cell potential for this reaction at . v In this zinc concentration cell, th spontaneously to transfer
Consider the following reaction at . Which of the following statements are correct? Choose all that apply. mol electrons The reaction is reactant-favored. delta