Home /
Expert Answers /
Chemistry /
a-compound-with-the-empirical-formula-mathrm-ch-4-mathrm-o-2-has-a-molecular-mass-of-pa501
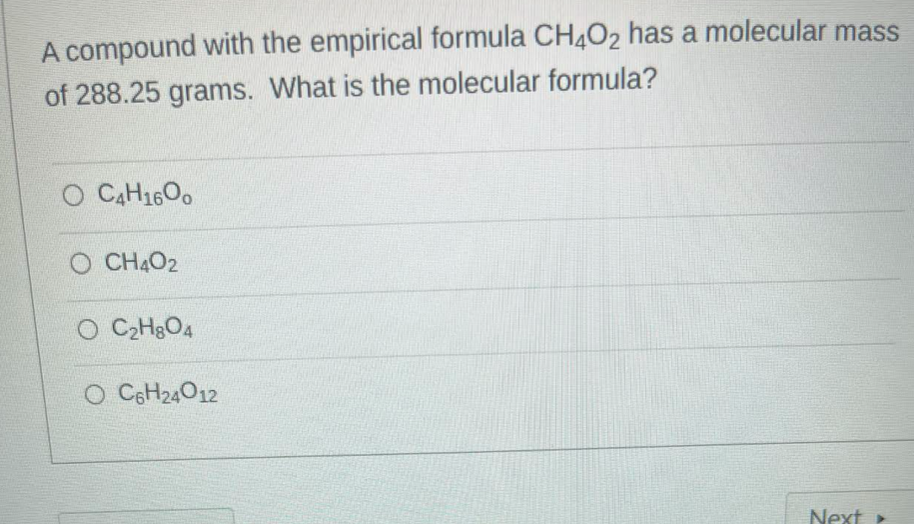
(Solved): A compound with the empirical formula \( \mathrm{CH}_{4} \mathrm{O}_{2} \) has a molecular mass of ...
A compound with the empirical formula \( \mathrm{CH}_{4} \mathrm{O}_{2} \) has a molecular mass of \( 288.25 \) grams. What is the molecular formula? \( \mathrm{C}_{4} \mathrm{H}_{16} \mathrm{O}_{0} \) \( \mathrm{CH}_{4} \mathrm{O}_{2} \) \( \mathrm{C}_{2} \mathrm{H}_{8} \mathrm{O}_{4} \) \( \mathrm{C}_{6} \mathrm{H}_{24} \mathrm{O}_{12} \)