Home /
Expert Answers /
Chemistry /
a-chemical-engineer-is-studying-the-following-reaction-h2-9-12-9-2-hi-9-at-the-temperature-pa979
(Solved): A chemical engineer is studying the following reaction: H2(9) + 12(9) 2 HI(9) At the temperature ...
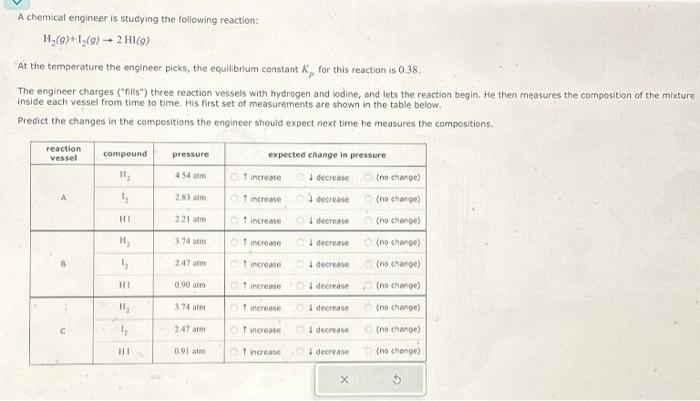
A chemical engineer is studying the following reaction:
H2(9) + 12(9) ? 2 HI(9)
At the temperature the engineer picks, the equilibrium constant kp, for this reaction is 0.38.
The engineer charges ("fills") three reaction vessels with hydrogen and iodine, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below.
Predict the changes in the compositions the engineer should expect next time he measures the compositions.
A chemical enginear is studying the following reaction: At the temperature the engineer picks, the equilibrium constant for this reaction is 0.38 . The engineer charges three reaction vessels with hydrogen and lodine, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions.