Home /
Expert Answers /
Chemistry /
a-certain-half-reaction-has-a-standard-reduction-potential-lred0-0-58v-an-engineer-propo-pa987
(Solved): A certain half-reaction has a standard reduction potential Lred0=0.58V. An engineer propo ...
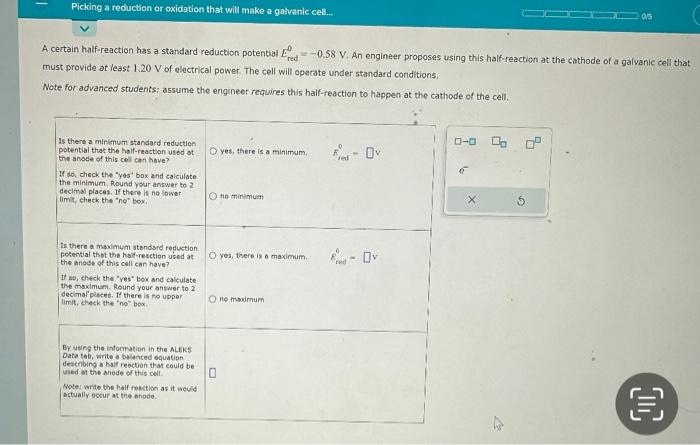
A certain half-reaction has a standard reduction potential . An engineer proposes using this half-reaction at the cathode of a galvanic cell that must provide at least 1,20 of electrical power. The cell will ananate vindar atandacd.
Expert Answer
To calculate the minimum standard reduction potential that the half-reaction used at the anode of this cell can have, we need to use the equation:E°cell = E°cathode - E°anodewhere E°cell is the standard cell potential, E°cathode is the standard reduction potential of the half-reaction used at the cathode, and E°anode is the standard reduction potential of the half-reaction used at the anode.We know that the E°cathode is -0.58 V and the E°cell must be at least 1.20 V. Therefore, we can rearrange the equation above to solve for the minimum E°anode:E°anode = E°cathode - E°cellE°anode = -0.58 V - (1.20 V)E°anode = -1.78 VTherefore, the minimum standard reduction potential that the half-reaction used at the anode of this cell can have is -1.78 V.Answer: Yes, the minimum standard reduction potential that the half-reaction used at the anode of this cell can have is E° = -1.78 V.