Home /
Expert Answers /
Chemistry /
a-calculate-the-values-of-the-rate-constant-k-assuming-that-the-reaction-is-first-order-what-is-t-pa790
(Solved): (A)Calculate the values of the rate constant k assuming that the reaction is first order. What is t ...
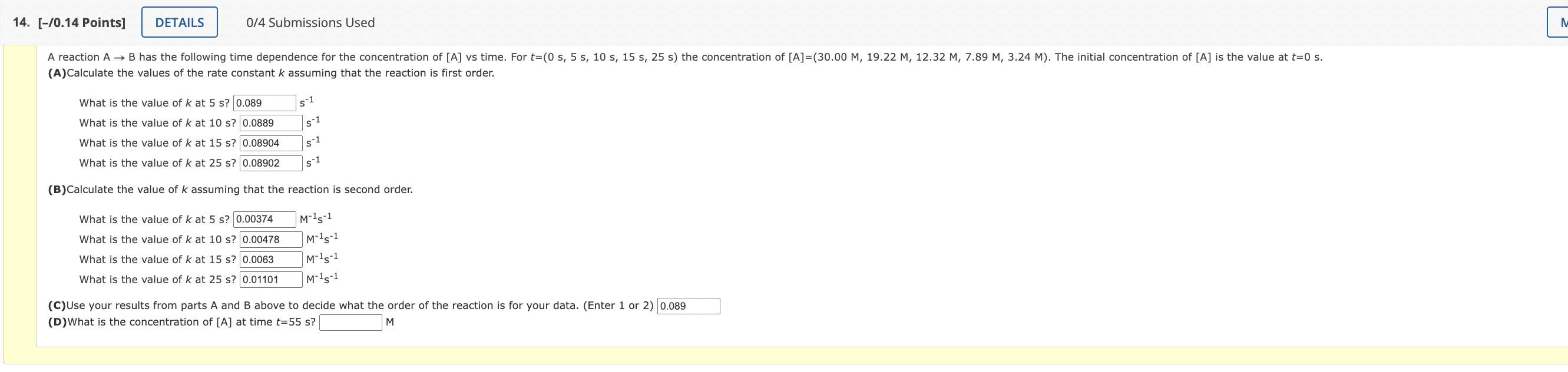
(A)Calculate the values of the rate constant assuming that the reaction is first order. What is the value of at ? What is the value of at ? What is the value of at ? What is the value of at ? 0.08902 (B)Calculate the value of assuming that the reaction is second order. What is the value of at ? What is the value of at ? What is the value of at ? What is the value of at 25 s? (C)Use your results from parts and above to decide what the order of the reaction is for your data. (Enter 1 or 2 ) 0.089 (D)What is the concentration of at time ? 1