Home /
Expert Answers /
Chemistry /
a-calculate-the-molar-solubility-of-silver-thiocyanate-agscn-in-pure-water-ksp-1-01012-pa123
(Solved): a Calculate the molar solubility of silver thiocyanate, AgSCN, in pure water (Ksp=1.01012). ...
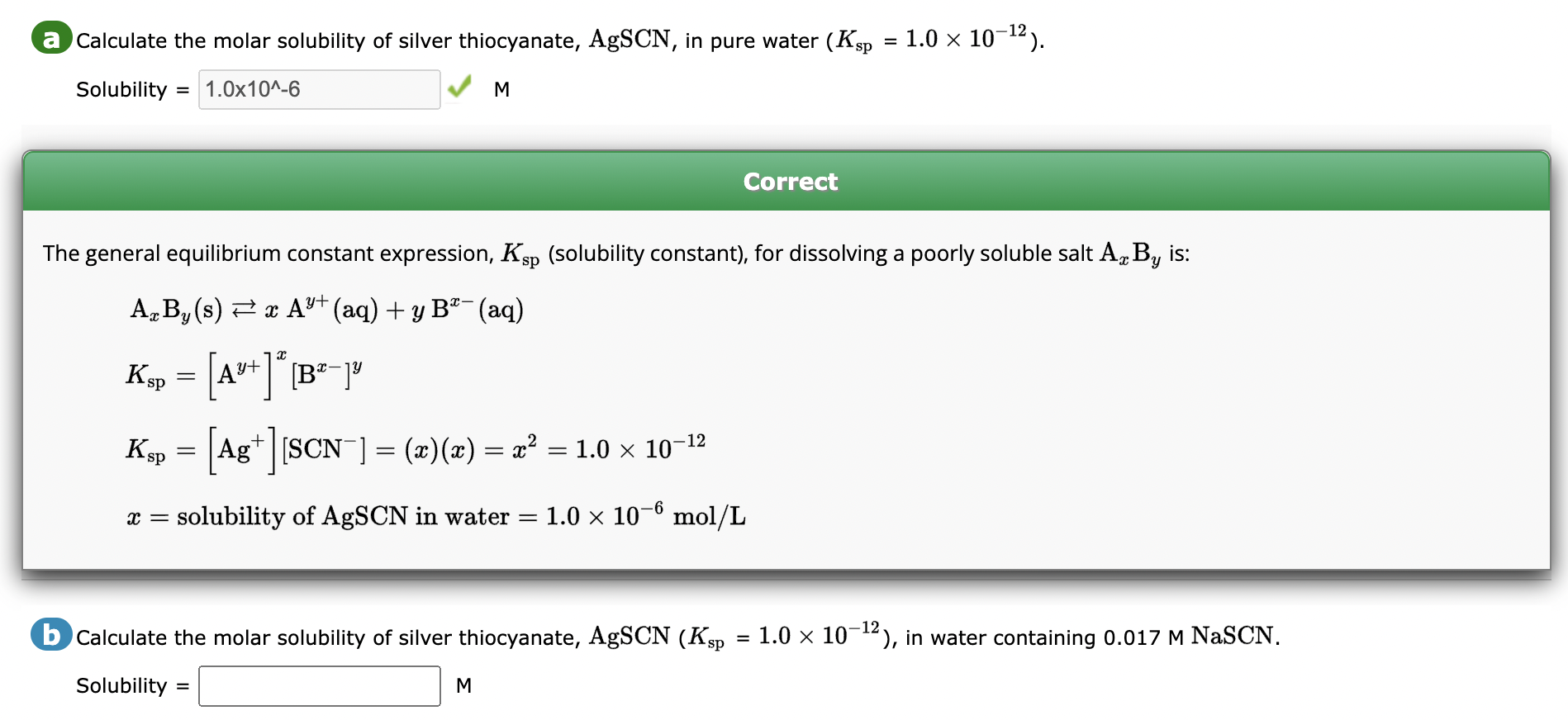
a Calculate the molar solubility of silver thiocyanate, , in pure water . Solubility M Correct The general equilibrium constant expression, (solubility constant), for dissolving a poorly soluble salt is: b Calculate the molar solubility of silver thiocyanate, , in water containing . Solubility M