Home /
Expert Answers /
Chemistry /
a-b-c-atomic-mass-is-a-weighted-average-of-the-masses-of-the-naturally-occurring-isotopes-of-that-pa224
(Solved): a) b)c) Atomic mass is a weighted average of the masses of the naturally occurring isotopes of that ...
a)
b)
c)
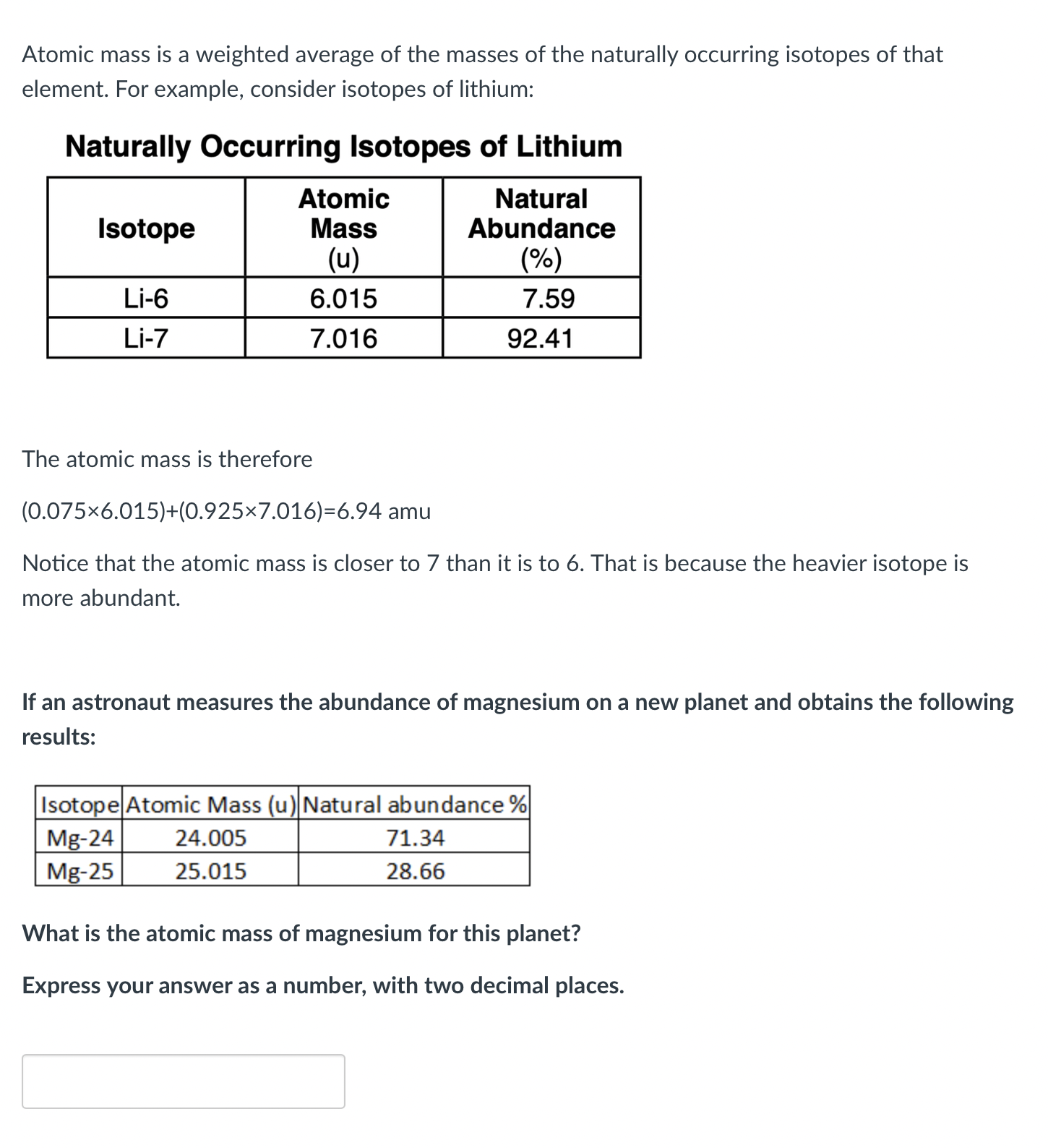
Atomic mass is a weighted average of the masses of the naturally occurring isotopes of that element. For example, consider isotopes of lithium: Naturally Occurring Isotopes of Lithium The atomic mass is therefore Notice that the atomic mass is closer to 7 than it is to 6 . That is because the heavier isotope is more abundant. If an astronaut measures the abundance of magnesium on a new planet and obtains the following results: What is the atomic mass of magnesium for this planet? Express your answer as a number, with two decimal places.
Which one of the following pairs of elements will have similar chemical properties? lithium and rubidium silicon and astatine phosphorus and sulfur aluminum and silver
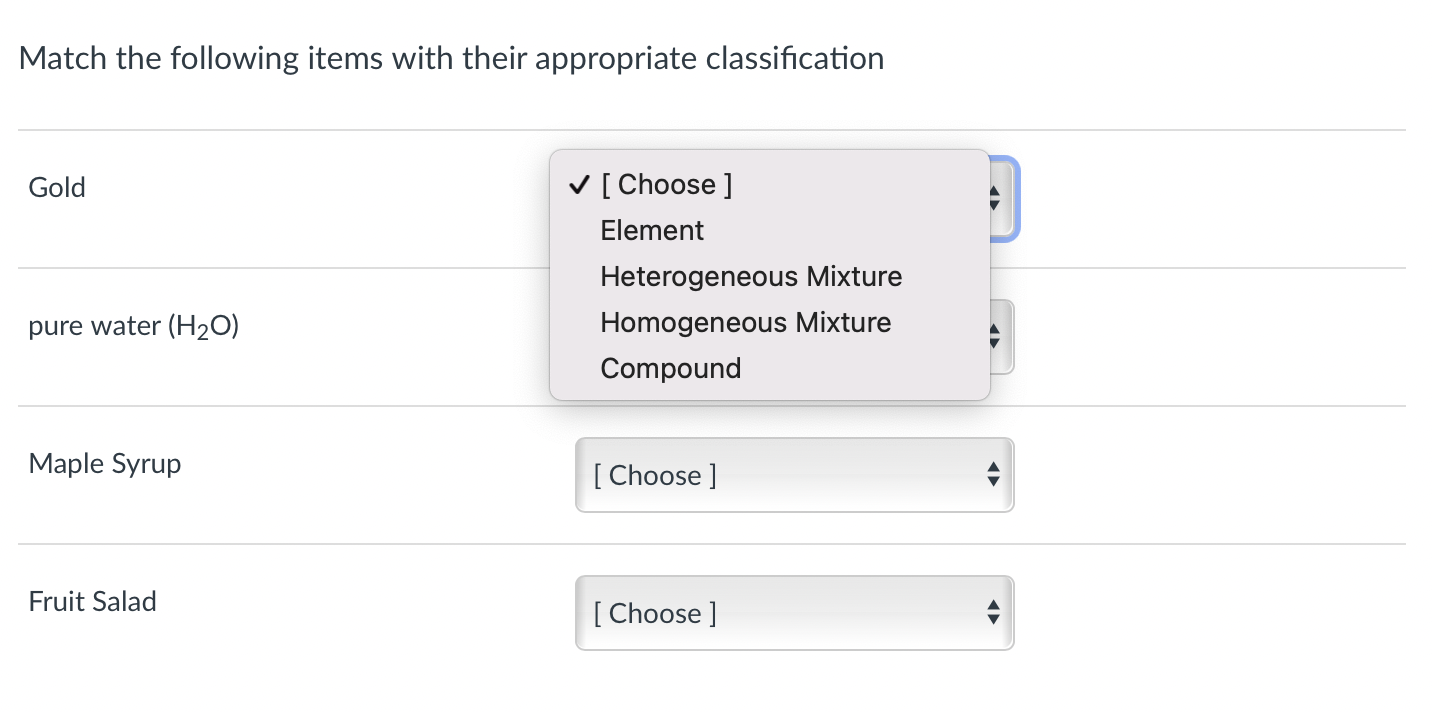
Match the following items with their appropriate classification Gold pure water Maple Syrup Fruit Salad