Home /
Expert Answers /
Chemical Engineering /
a-a-possible-mechanism-for-the-hydrogenation-of-ethylene-mathrm-c-2-mathrm-h-4-mat-pa826
(Solved): a) A possible mechanism for the hydrogenation of ethylene, \( \mathrm{C}_{2} \mathrm{H}_{4}+\mat ...
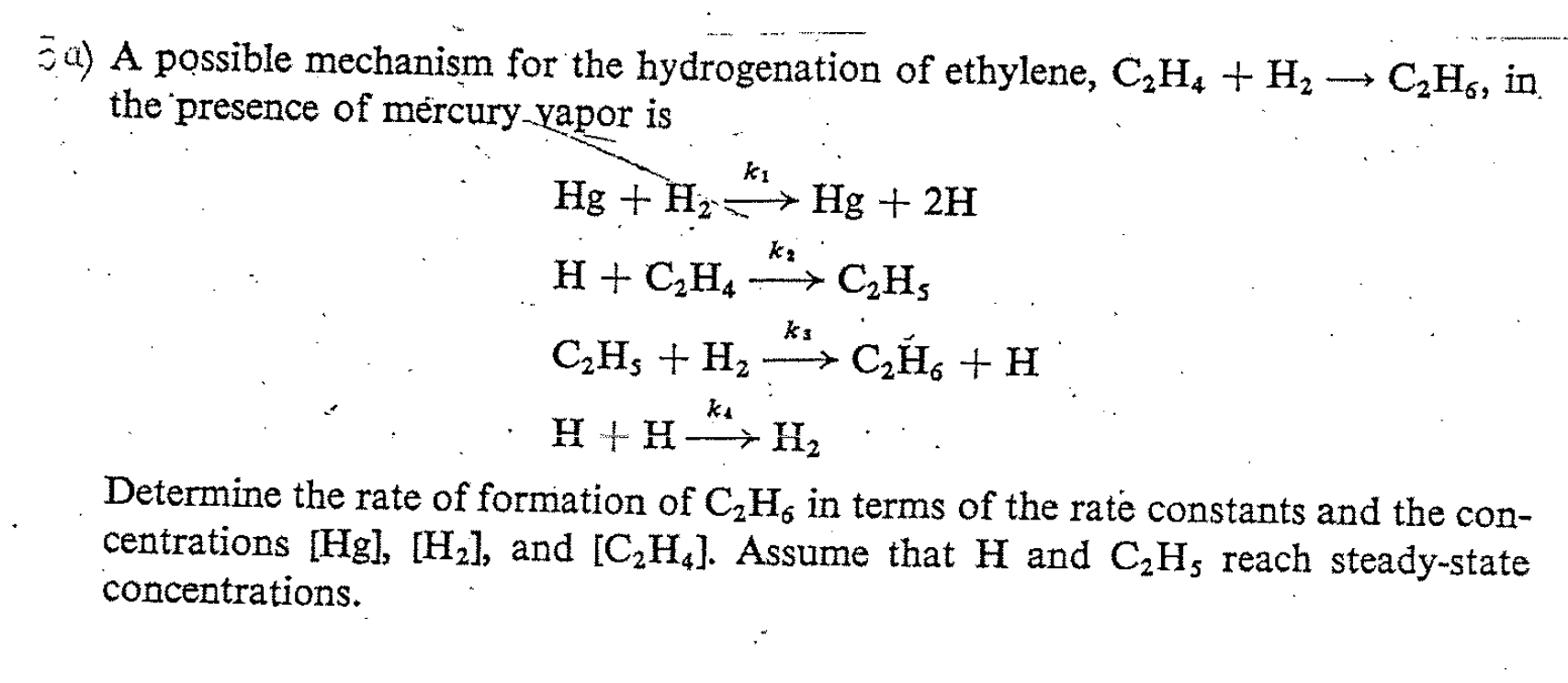
?a) A possible mechanism for the hydrogenation of ethylene, \( \mathrm{C}_{2} \mathrm{H}_{4}+\mathrm{H}_{2} \rightarrow \mathrm{C}_{2} \mathrm{H}_{6} \), in the presence of mercury yapor is \[ \begin{array}{l} \mathrm{Hg}+\mathrm{H}_{2} \stackrel{k_{1}}{\longrightarrow} \mathrm{Hg}+2 \mathrm{H} \\ \mathrm{H}+\mathrm{C}_{2} \mathrm{H}_{4} \stackrel{k_{2}}{\longrightarrow} \mathrm{C}_{2} \mathrm{H}_{5} \\ \mathrm{C}_{2} \mathrm{H}_{5}+\mathrm{H}_{2} \stackrel{k_{3}}{\longrightarrow} \mathrm{C}_{2} \breve{\mathrm{H}}_{6}+\mathrm{H} \\ \mathrm{H}+\mathrm{H} \stackrel{k_{1}}{\longrightarrow} \mathrm{H}_{2} \end{array} \] Determine the rate of formation of \( \mathrm{C}_{2} \mathrm{H}_{6} \) in terms of the rate constants and the concentrations \( [\mathrm{Hg}],\left[\mathrm{H}_{2}\right] \), and \( \left[\mathrm{C}_{2} \mathrm{H}_{4}\right] \). Assume that \( \mathrm{H} \) and \( \mathrm{C}_{2} \mathrm{H}_{5} \) reach steady-state concentrations.
Expert Answer
using rate law ans steady s