Home /
Expert Answers /
Chemistry /
a-25-0-mathrm-ml-sample-of-a-solution-of-a-monoprotic-acid-is-titrated-with-a-0-115-ma-pa503
(Solved): A \( 25.0 \mathrm{~mL} \) sample of a solution of a monoprotic acid is titrated with a \( 0.115 \ma ...
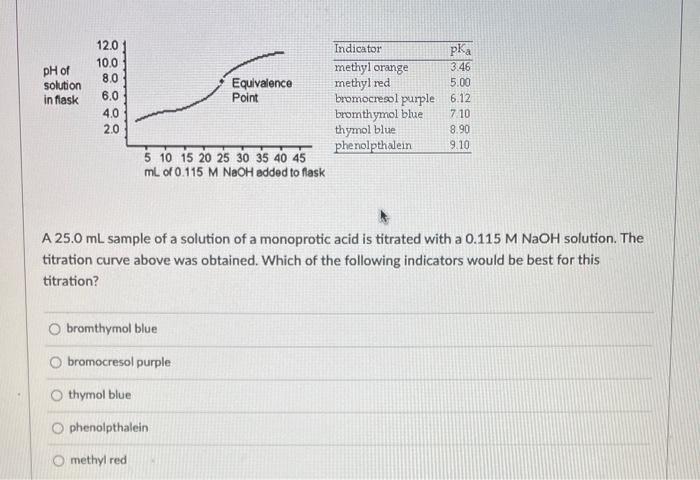

A \( 25.0 \mathrm{~mL} \) sample of a solution of a monoprotic acid is titrated with a \( 0.115 \mathrm{M} \mathrm{NaOH} \) solution. The titration curve above was obtained. Which of the following indicators would be best for this titration? bromthymol blue bromocresol purple thymol blue phenolpthalein methyl red
A \( 25.0 \mathrm{~mL} \) sample of a solution of an unknown compound is titrated with a \( 0.115 \mathrm{M} \mathrm{NaOH} \) solution. The titration curve above was obtained. The unknown compound is a strong acid a strong base neither an acid nor a base a weak base a weak acid
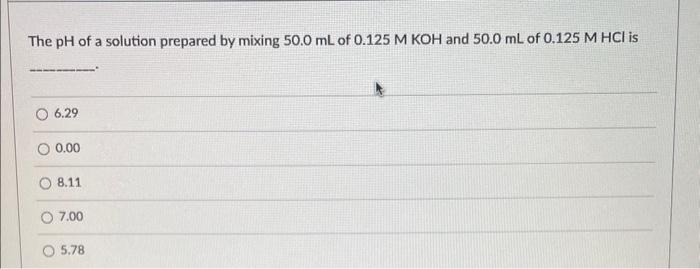
The \( \mathrm{pH} \) of a solution prepared by mixing \( 50.0 \mathrm{~mL} \) of \( 0.125 \mathrm{M} \mathrm{KOH} \) and \( 50.0 \mathrm{~mL} \) of \( 0.125 \mathrm{M} \mathrm{HCl} \) is \( 6.29 \) \( 0.00 \) \( 8.11 \) \( 7.00 \) \( 5.78 \)
Expert Answer
1) The pH at equivalence point