Home /
Expert Answers /
Chemistry /
a-15-0-mathrm-ml-sample-of-whiskey-was-diluted-to-500-0-mathrm-ml-a-3-00-ma-pa863
(Solved): A \( 15.0 \mathrm{~mL} \) sample of whiskey was diluted to \( 500.0 \mathrm{~mL} \). A \( 3.00 \ma ...
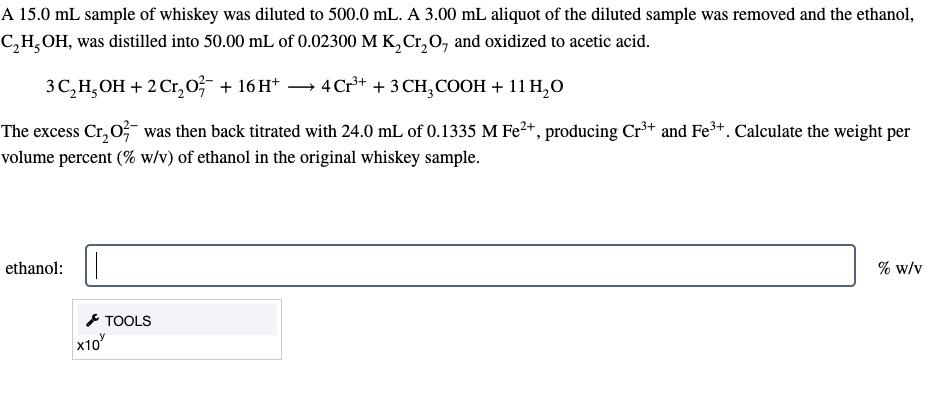
A \( 15.0 \mathrm{~mL} \) sample of whiskey was diluted to \( 500.0 \mathrm{~mL} \). A \( 3.00 \mathrm{~mL} \) aliquot of the diluted sample was removed and the ethanol, \( \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH} \), was distilled into \( 50.00 \mathrm{~mL} \) of \( 0.02300 \mathrm{M} \mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7} \) and oxidized to acetic acid. \[ 3 \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}+2 \mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+16 \mathrm{H}^{+} \longrightarrow 4 \mathrm{Cr}^{3+}+3 \mathrm{CH}_{3} \mathrm{COOH}+11 \mathrm{H}_{2} \mathrm{O} \] The excess \( \mathrm{Cr}_{2} \mathrm{O}_{7}^{2-} \) was then back titrated with \( 24.0 \mathrm{~mL} \) of \( 0.1335 \mathrm{M} \mathrm{Fe}^{2+} \), producing \( \mathrm{Cr}^{3+} \) and \( \mathrm{Fe}^{3+} \). Calculate the weight per volume percent (\% w/v) of ethanol in the original whiskey sample. ethanol: