Home /
Expert Answers /
Chemistry /
a-100-0ml-solution-containing-0-976g-of-maleic-acid-mw-116-072g-mol-is-titrated-with-0-366m-pa343
(Solved): A 100.0mL solution containing 0.976g of maleic acid (MW=116.072g/mol) is titrated with 0.366M ...
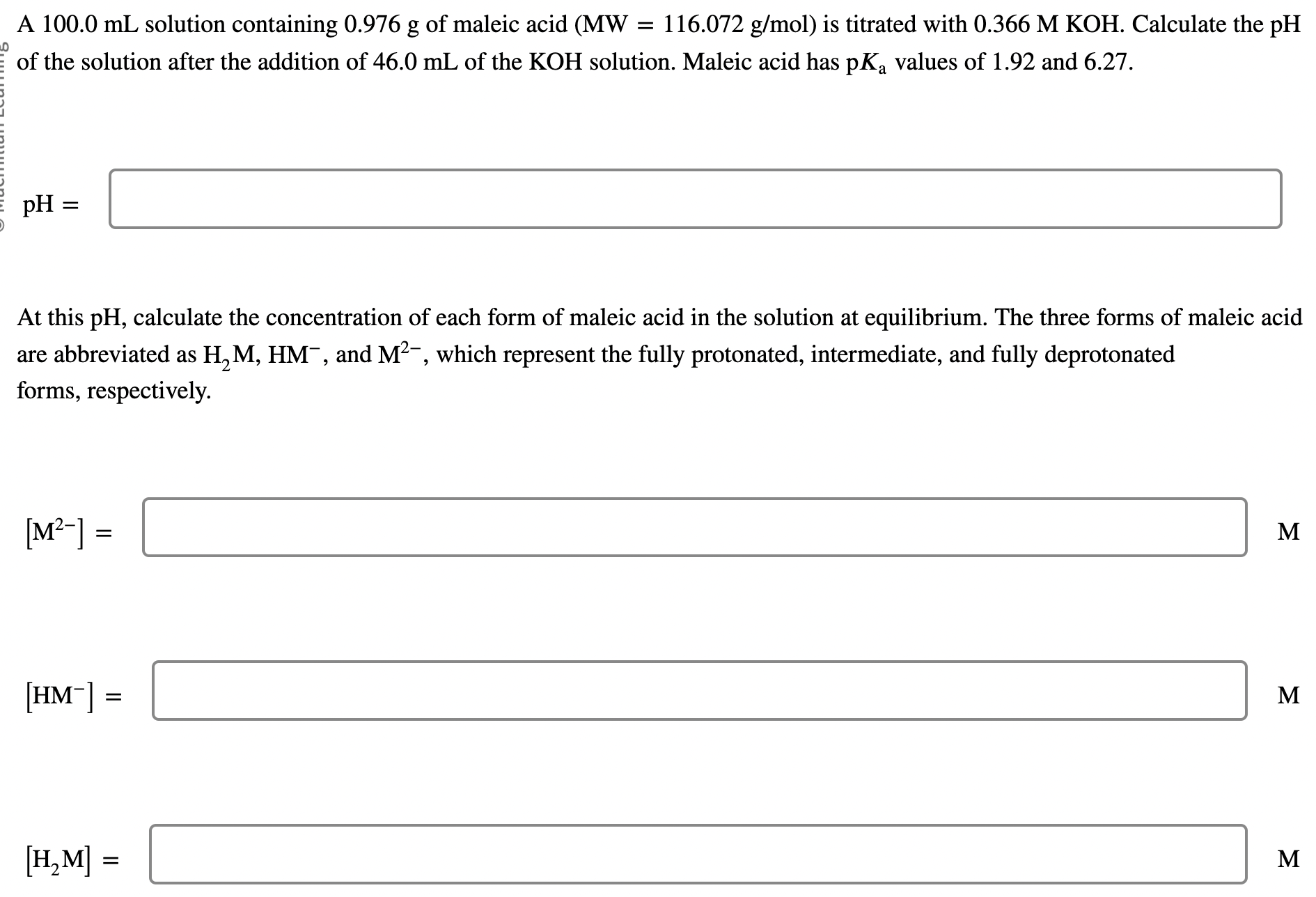
A solution containing of maleic acid is titrated with . Calculate the of the solution after the addition of of the solution. Maleic acid has values of 1.92 and 6.27. At this , calculate the concentration of each form of maleic acid in the solution at equilibrium. The three forms of maleic acid are abbreviated as , and , which represent the fully protonated, intermediate, and fully deprotonated forms, respectively. M M M