Home /
Expert Answers /
Chemistry /
a-1-44-l-buffer-solution-consists-of-0-289m-propanoic-acid-and-0-189m-sodium-propanoate-calculate-pa220
(Solved): A 1.44 L buffer solution consists of 0.289M propanoic acid and 0.189M sodium propanoate. Calculate ...
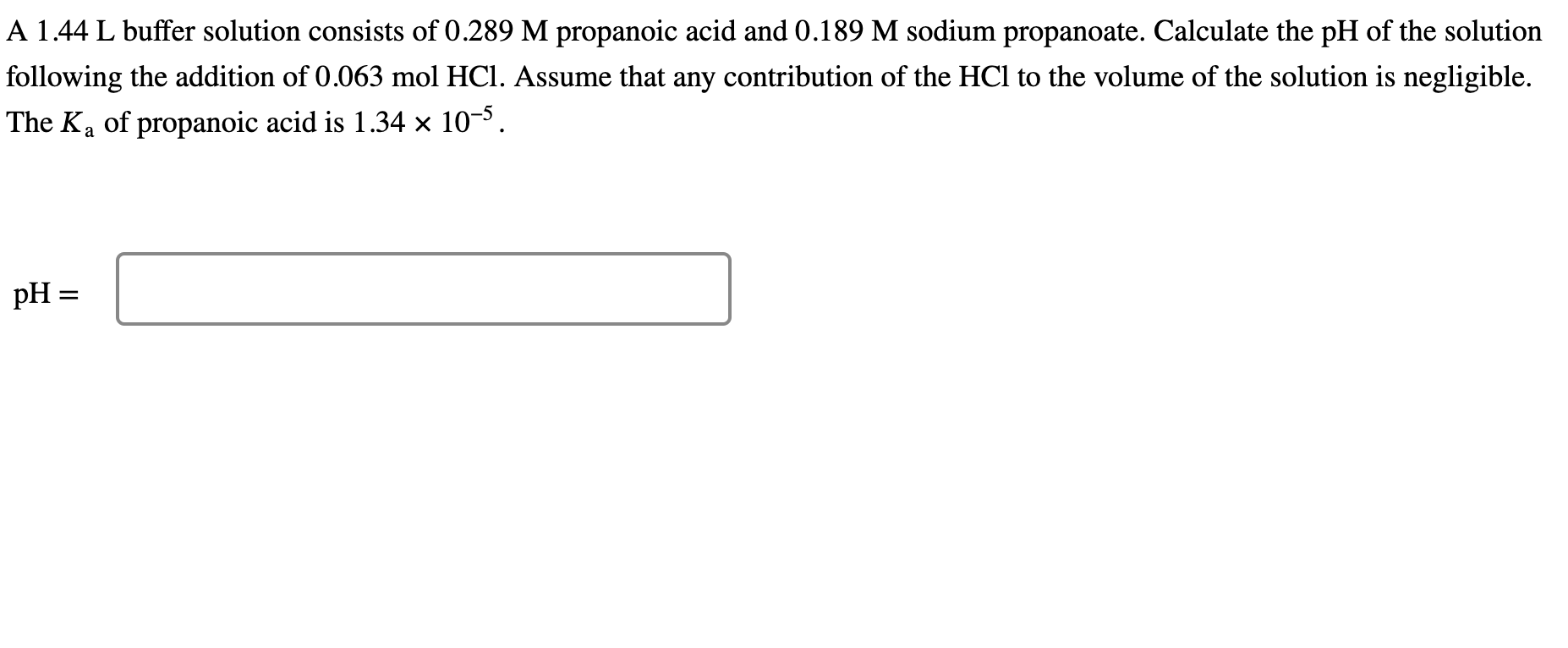
A 1.44 L buffer solution consists of propanoic acid and sodium propanoate. Calculate the of the solution following the addition of . Assume that any contribution of the to the volume of the solution is negligible. The of propanoic acid is .