Home /
Expert Answers /
Chemistry /
a-0-748m-solution-of-a-weak-acid-ha-is-made-the-solution-has-a-ph-of-3-75-calculate-the-ka-pa387
(Solved): A 0.748M solution of a weak acid (HA) is made. The solution has a pH of 3.75. Calculate the Ka ...
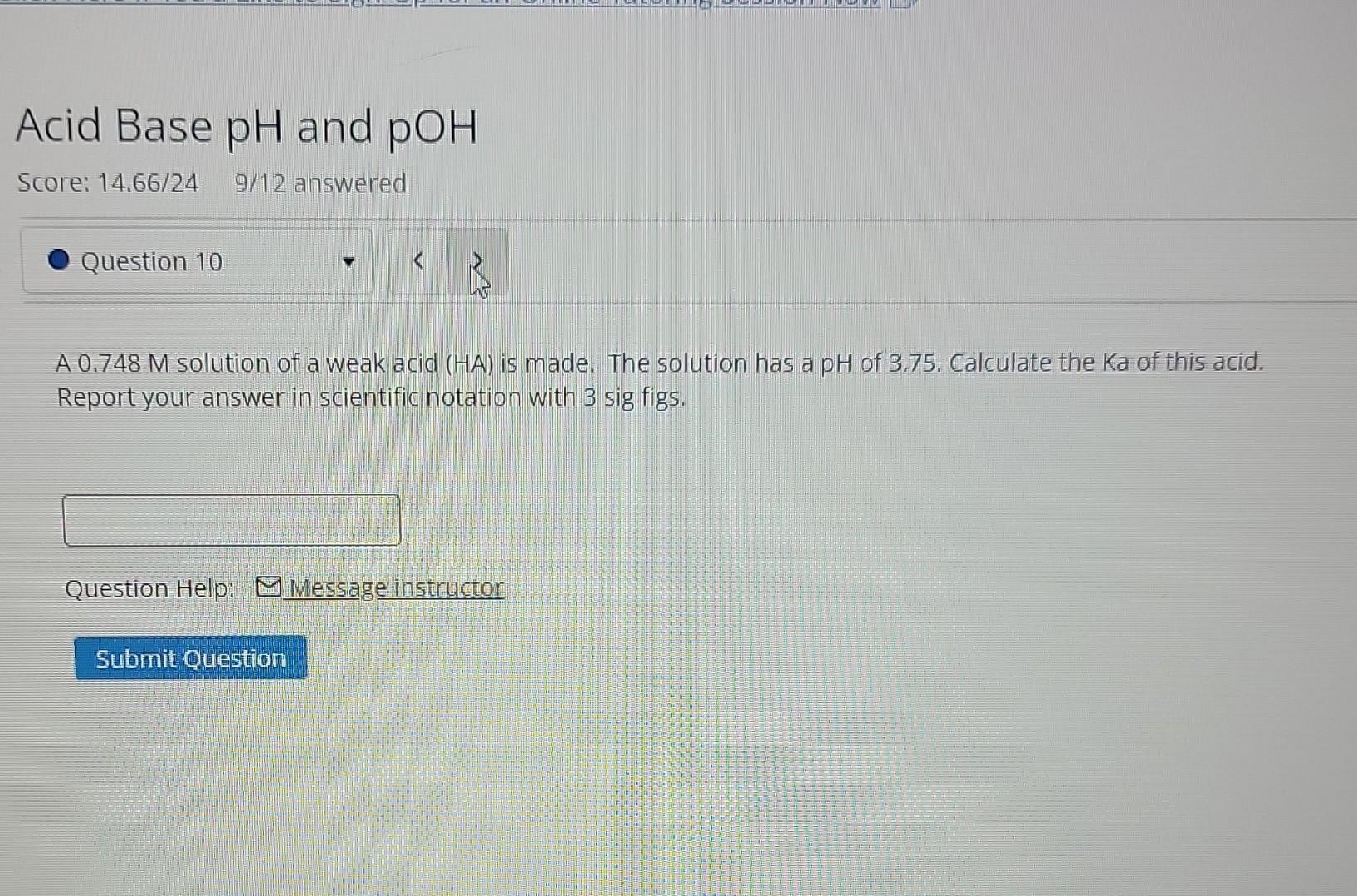
A solution of a weak acid (HA) is made. The solution has a pH of 3.75. Calculate the Ka of this acid. Report your answer in scientific notation with 3 sig figs. Question Help: Message instructor