Home /
Expert Answers /
Chemistry /
a-0-236-mathrm-g-piece-of-solid-magnesium-reacts-with-gaseous-arysen-from-the-atmosphere-t-pa183
(Solved): A \( 0.236 \mathrm{~g} \) piece of solid magnesium reacts with gaseous arysen from the atmosphere t ...
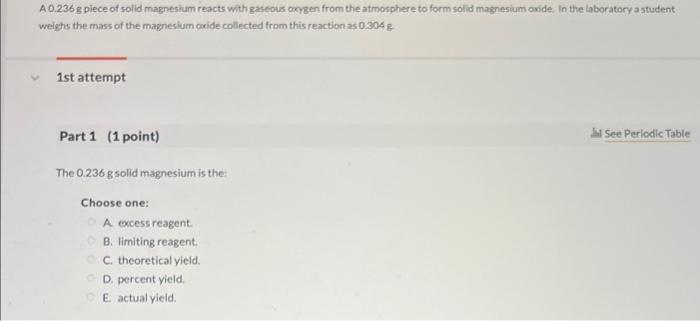

A \( 0.236 \mathrm{~g} \) piece of solid magnesium reacts with gaseous arysen from the atmosphere to form solid magnesium oxide in the laboratory a student weighs the mass of the magnesium oxide collected from this reaction as \( 0304 \mathrm{~g} \) 1st attempt Part 1 (1 point) The \( 0.236 \mathrm{~g} \) solid magnesium is the: Choose one: A excess reagent. B. limiting reagent. C. theoretical yieid. D. percent yield. E. actual yield.
The \( 0.304 \mathrm{~g} \) magnesium oxide is the: Choose one: A. percent yield. B. theoretical yield. C. limiting reagent. D. actual yield. E. excess reagent.
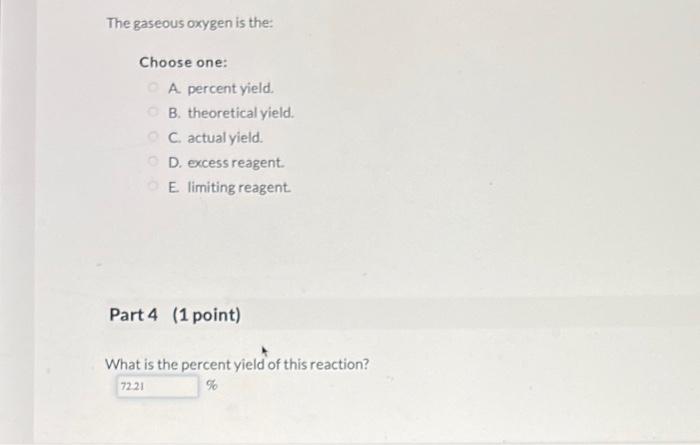
A. percent yield. B. theoretical yield. C. actual yield. D. excess reagent. E. limiting reagent. Part 4 (1 point) What is the percent yield of this reaction?