Home /
Expert Answers /
Chemistry /
9-the-density-of-a-solution-of-potassium-hydroxide-is-1-4791-mathrm-g-mathrm-cc-and-pa915
(Solved): 9. The density of a solution of potassium hydroxide is \( 1.4791 \mathrm{~g} / \mathrm{cc} \), and ...
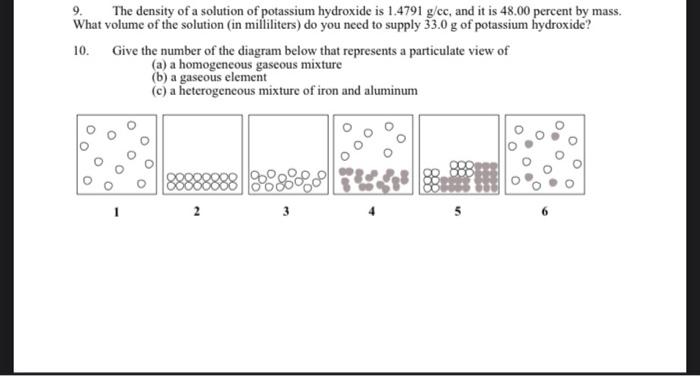
9. The density of a solution of potassium hydroxide is \( 1.4791 \mathrm{~g} / \mathrm{cc} \), and it is \( 48.00 \) percent by mass. What volume of the solution (in milliliters) do you need to supply \( 33.0 \mathrm{~g} \) of potassium hydroxide? 10. Give the number of the diagram below that represents a particulate view of (a) a homogeneous gaseous mixture (b) a gaseous element (c) a heterogeneous mixture of iron and aluminum
Expert Answer
The density of the solution of KOH ( potassium hydroxide ) is 1.4791 g / cc It is 48% by mass. it means, 100 g of solution contains 48 g of KOH.