Home /
Expert Answers /
Chemistry /
9-hydrate-is-the-function-group-made-when-water-adds-into-a-ketone-or-aldehyde-under-acidic-condi-pa805
(Solved): 9. Hydrate is the function group made when water adds into a ketone or aldehyde under acidic condi ...
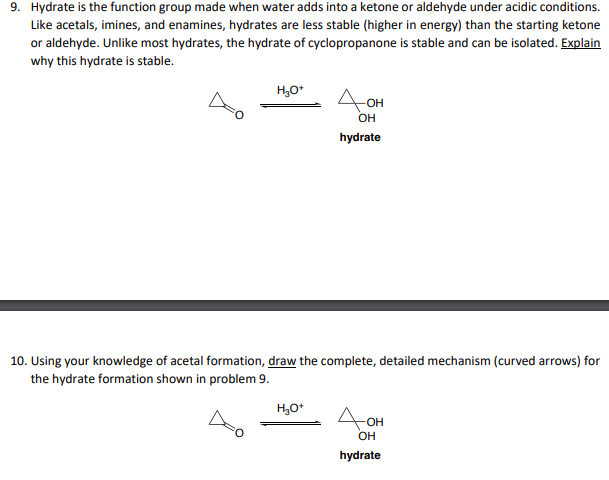
9. Hydrate is the function group made when water adds into a ketone or aldehyde under acidic conditions. Like acetals, imines, and enamines, hydrates are less stable (higher in energy) than the starting ketone or aldehyde. Unlike most hydrates, the hydrate of cyclopropanone is stable and can be isolated. Explain why this hydrate is stable. hydrate 10. Using your knowledge of acetal formation, draw the complete, detailed mechanism (curved arrows) for the hydrate formation shown in problem 9. hydrate
Expert Answer
Answer- 9) The product Hydrate of cyclopropanone has lesser bond angle strain tha