Home /
Expert Answers /
Chemical Engineering /
9-a-explain-the-atomic-hard-sphere-model-b-what-is-the-coordination-number-in-a-bcc-structure-pa928
(Solved): 9. (a) Explain the atomic hard sphere model. (b) What is the coordination number in a BCC structure ...
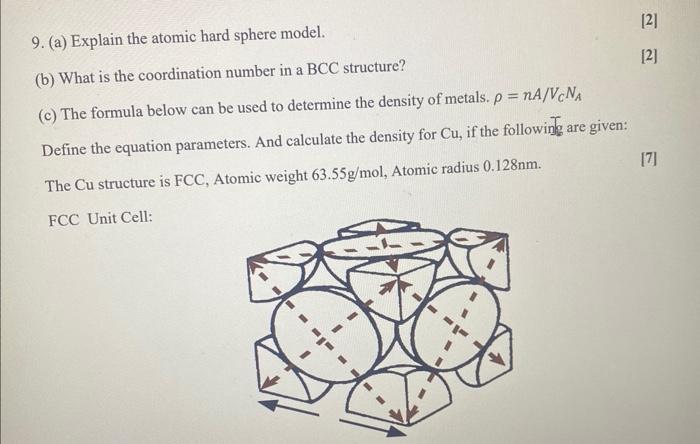
9. (a) Explain the atomic hard sphere model. (b) What is the coordination number in a BCC structure? (c) The formula below can be used to determine the density of metals. \( \rho=n A / V_{C} N_{A} \) Define the equation parameters. And calculate the density for \( \mathrm{Cu} \), if the following are given: The \( \mathrm{Cu} \) structure is \( \mathrm{FCC} \), Atomic weight \( 63.55 \mathrm{~g} / \mathrm{mol} \), Atomic radius \( 0.128 \mathrm{~nm} \). [7] FCC Unit Cell:
Expert Answer
Hi here is the solution for your question. I hope it will help you if anything is not clear to you or I am wrong anywhere please let me know before giving your valuable feedback. I would like to im