Home /
Expert Answers /
Chemical Engineering /
9-63-methanol-vapor-is-burned-with-excess-air-in-a-catalytic-combustion-chamber-liquid-methanol-i-pa783
(Solved): 9.63. Methanol vapor is burned with excess air in a catalytic combustion chamber. Liquid methanol i ...
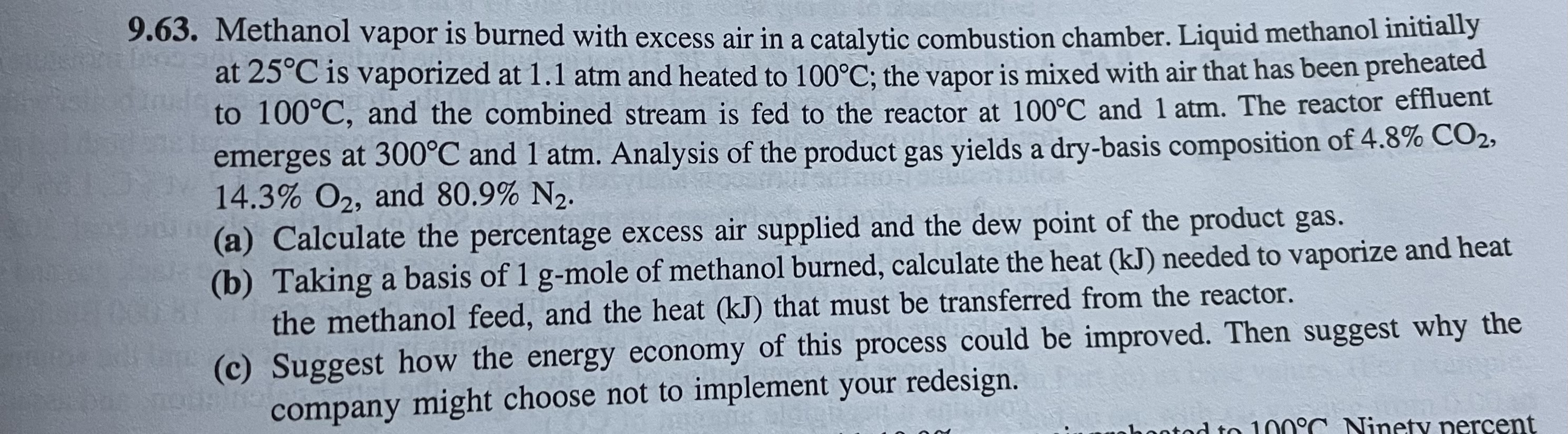
9.63. Methanol vapor is burned with excess air in a catalytic combustion chamber. Liquid methanol initially at is vaporized at and heated to ; the vapor is mixed with air that has been preheated to , and the combined stream is fed to the reactor at and . The reactor effluent emerges at and . Analysis of the product gas yields a dry-basis composition of , , and . (a) Calculate the percentage excess air supplied and the dew point of the product gas. (b) Taking a basis of -mole of methanol burned, calculate the heat needed to vaporize and heat the methanol feed, and the heat that must be transferred from the reactor. (c) Suggest how the energy economy of this process could be improved. Then suggest why the company might choose not to implement your redesign.