Home /
Expert Answers /
Chemistry /
9-3-enthalpy-participation-calculate-the-amount-of-heat-released-by-the-combustion-of-3-75-mol-of-b-pa857
(Solved): 9.3 ENTHALPY: PARTICIPATION Calculate the amount of heat released by the combustion of 3.75 mol of b ...
9.3 ENTHALPY: PARTICIPATION Calculate the amount of heat released by the combustion of 3.75 mol of benzene (CH). State whether the reaction is endothermic or exothermic and whether the reactants or the products contain more stored heat energy. 2C6H6+150??? 1200? + 6H?O ??= -98.0 kJ Identify each process as endothermic or exothermic and indicate the sign of AH. a) sweat evaporating from skin b) water freezing in a freezer c) wood burning in a fire d) an ice cube melting e) nail polish remover quickly evaporating after it is accidentally spilled on the skin f) gasoline burning within the cylinder of an automobile engine openstax
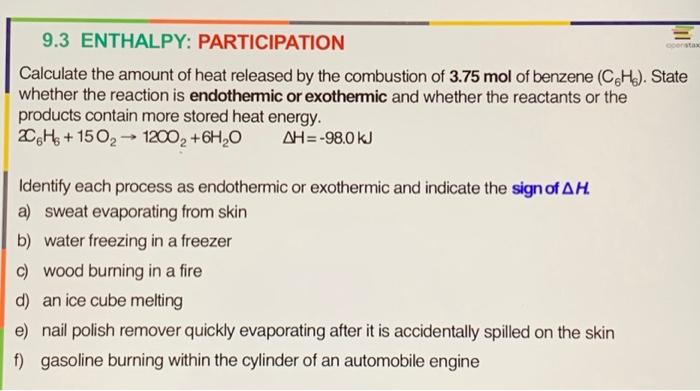
Calculate the amount of heat released by the combustion of of benzene . State whether the reaction is endothermic or exothermic and whether the reactants or the products contain more stored heat energy. Identify each process as endothermic or exothermic and indicate the sign of . a) sweat evaporating from skin b) water freezing in a freezer c) wood burning in a fire d) an ice cube melting e) nail polish remover quickly evaporating after it is accidentally spilled on the skin f) gasoline burning within the cylinder of an automobile engine
Expert Answer
To calculate the amount of heat released by the combustion of 3.75 mol of benzene (C6H6), we can use...