Home /
Expert Answers /
Chemistry /
6-the-reaction-chcl3-g-cl2-g-ccl4-g-hcl-g-occurs-according-to-the-mechani-pa849
(Solved): 6. The reaction CHCl3(g)+Cl2(g)CCl4(g)+HCl(g) occurs according to the mechani ...
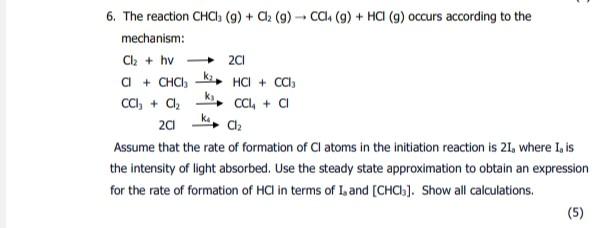
6. The reaction occurs according to the mechanism: Assume that the rate of formation of atoms in the initiation reaction is where is the intensity of light absorbed. Use the steady state approximation to obtain an expression for the rate of formation of in terms of and . Show all calculations.