Home /
Expert Answers /
Chemistry /
6-suggest-a-reasonable-mechanism-for-the-reaction-below-you-must-draw-the-curved-arrows-below-the-pa289
(Solved): 6. Suggest a reasonable mechanism for the reaction below. You must draw the curved arrows below the ...
6. Suggest a reasonable mechanism for the reaction below. You must draw the curved arrows below the equation given! (I pt) Hint: notice that this is an intramolecular cyclization.
7a. provide the major organic product for each box (via each transformation): (1.5 pts, 0.25 pt)
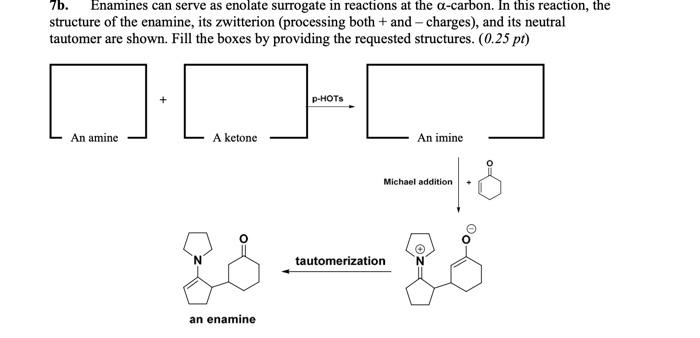
7b. Enamines can serve as enolate surrogate in reactions at the -carbon. In this reaction, the structure of the enamine, its zwitterion (processing both + and - charges), and its neutral tautomer are shown. Fill the boxes by providing the requested structures. (0.25 pt)
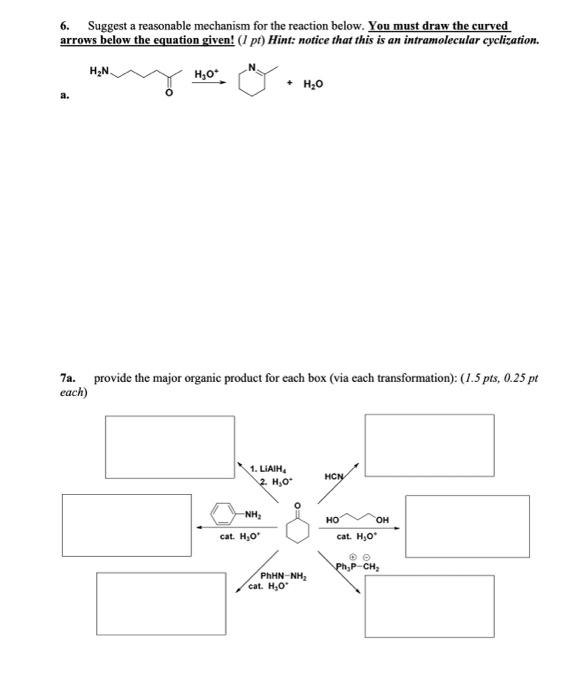
6. Suggest a reasonable mechanism for the reaction below. You must draw the curved arrows below the equation given! ( \( I \) pt) Hint: notice that this is an intramolecular cyclization. a. \( \stackrel{\mathrm{H}_{3} \mathrm{O}^{+}}{\longrightarrow} \) 7a. provide the major organic product for each box (via each transformation): ( \( 1.5 \mathrm{pts}, 0.25 \mathrm{pt} \) each)
7b. Enamines can serve as enolate surrogate in reactions at the \( \alpha \)-carbon. In this reaction, the structure of the enamine, its zwitterion (processing both \( + \) and - charges), and its neutral tautomer are shown. Fill the boxes by providing the requested structures. \( (0.25 \mathrm{pt}) \) tautomerization an enamine